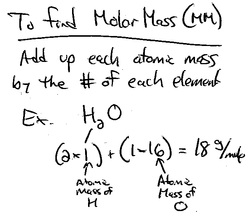
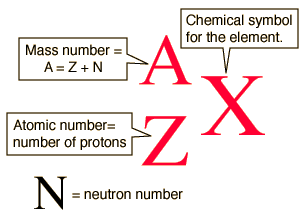How To Find The Mass Of An Element
Find the mass of oxygen in 1 mol of adrenaline.
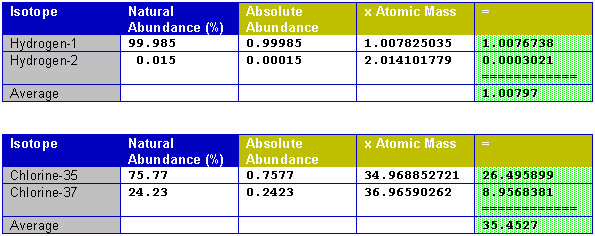
How to find the mass of an element. Massisotope n abundanceisotope n. To calculate atomic mass start by finding the atomic number of the element which is the number above the element on the periodic table. Find the weighted average of the atomic mass of its stable isotopes. The method used to find atomic mass depends on whether youre looking at a single atom a natural sample or a sample containing a known ratio of isotopes.
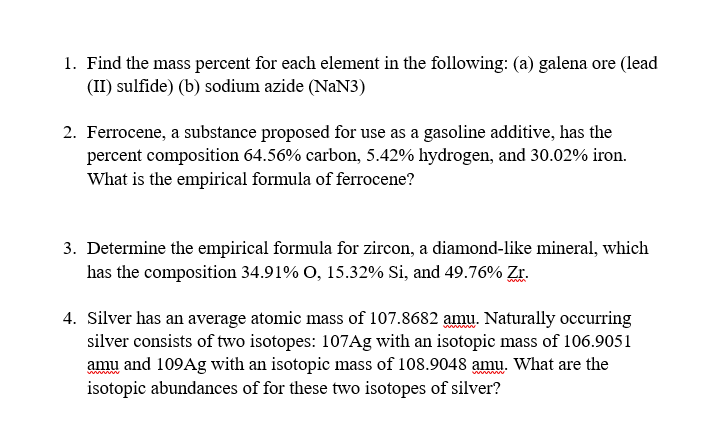
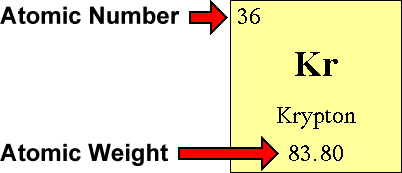
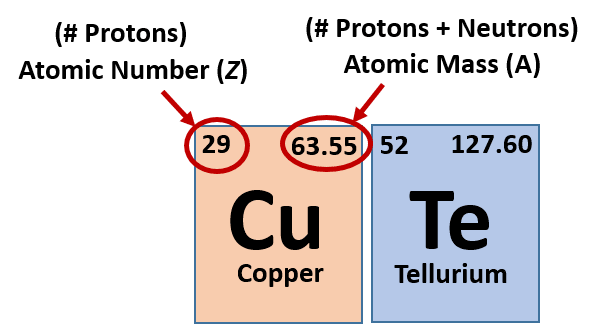
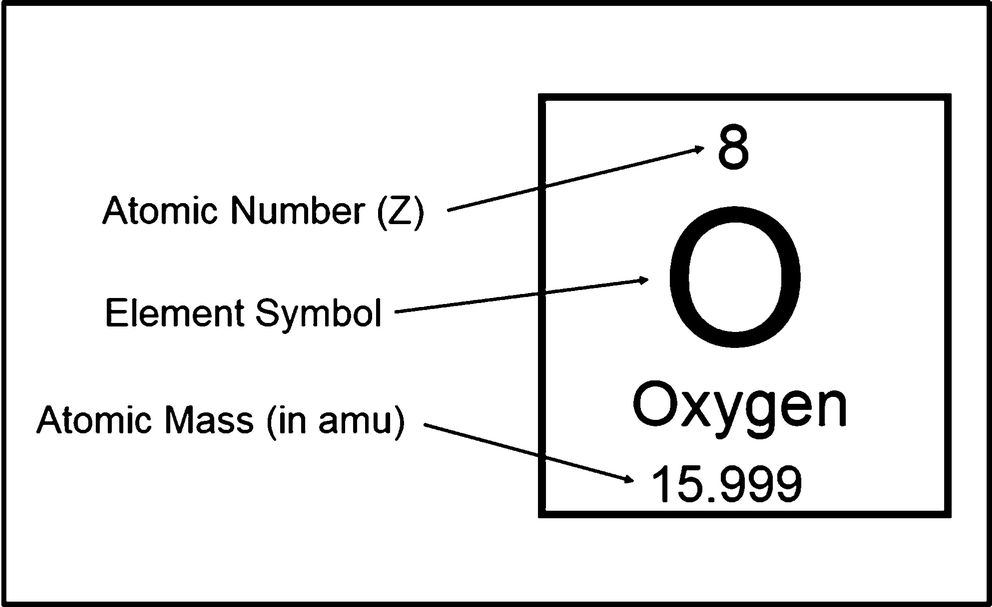
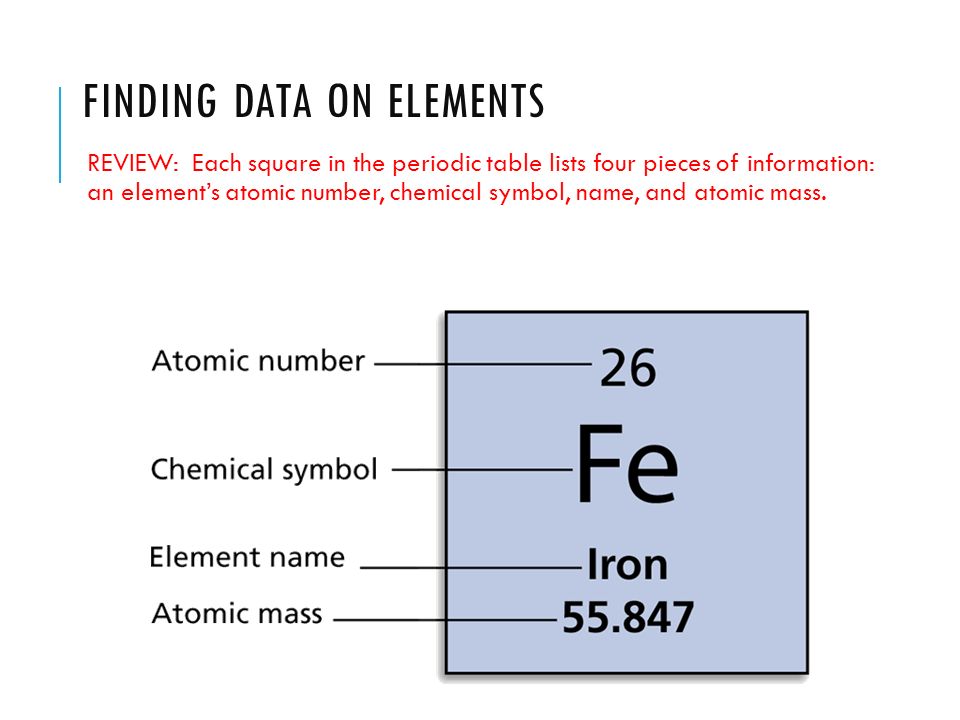
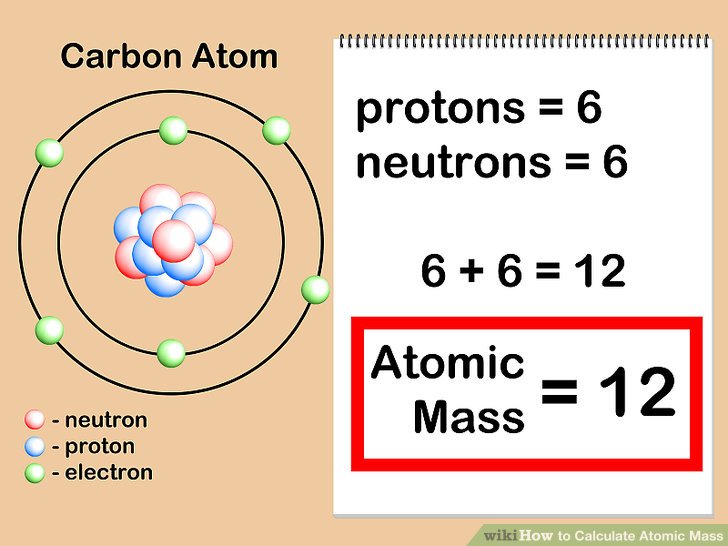
Finally add the atomic number and the number of neutrons to get the atomic mass. Next find the number of neutrons in the nucleus by subtracting the atomic number from the isotope number. The relative atomic mass scale is used to compare the masses of different atoms. The atomic mass number for each element is listed in atomic mass units under its symbol in the periodic table.
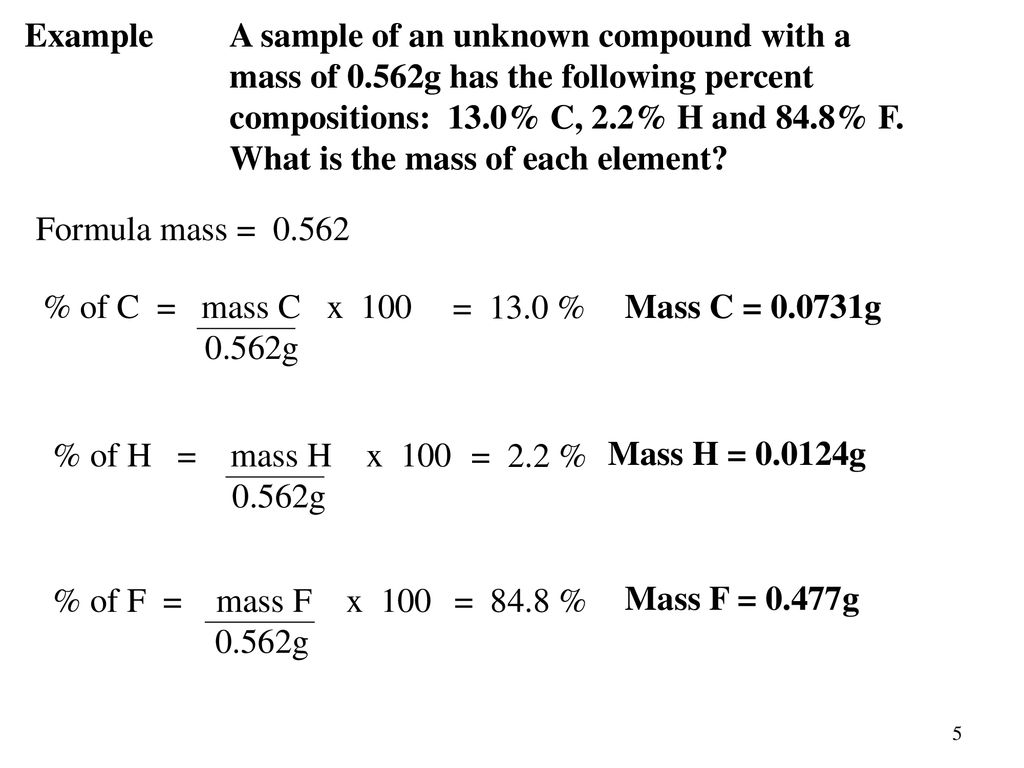
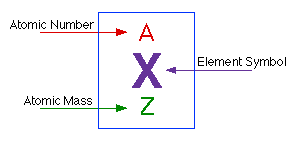
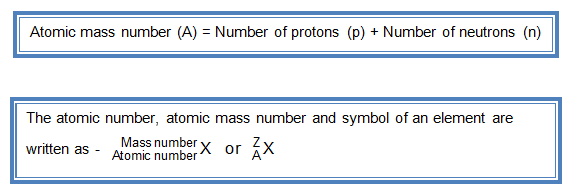
To do this you must need to know its chemical formula which is c x 9 h x 13. Calculate percentage composition of. An elements mass number a is the sum of the number of protons and the number of neutrons. The best place to look for an elements atomic mass number is in the periodic table.
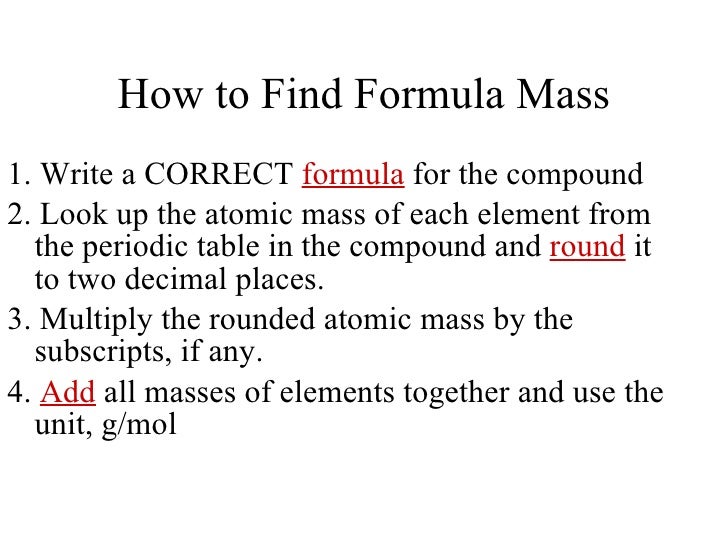
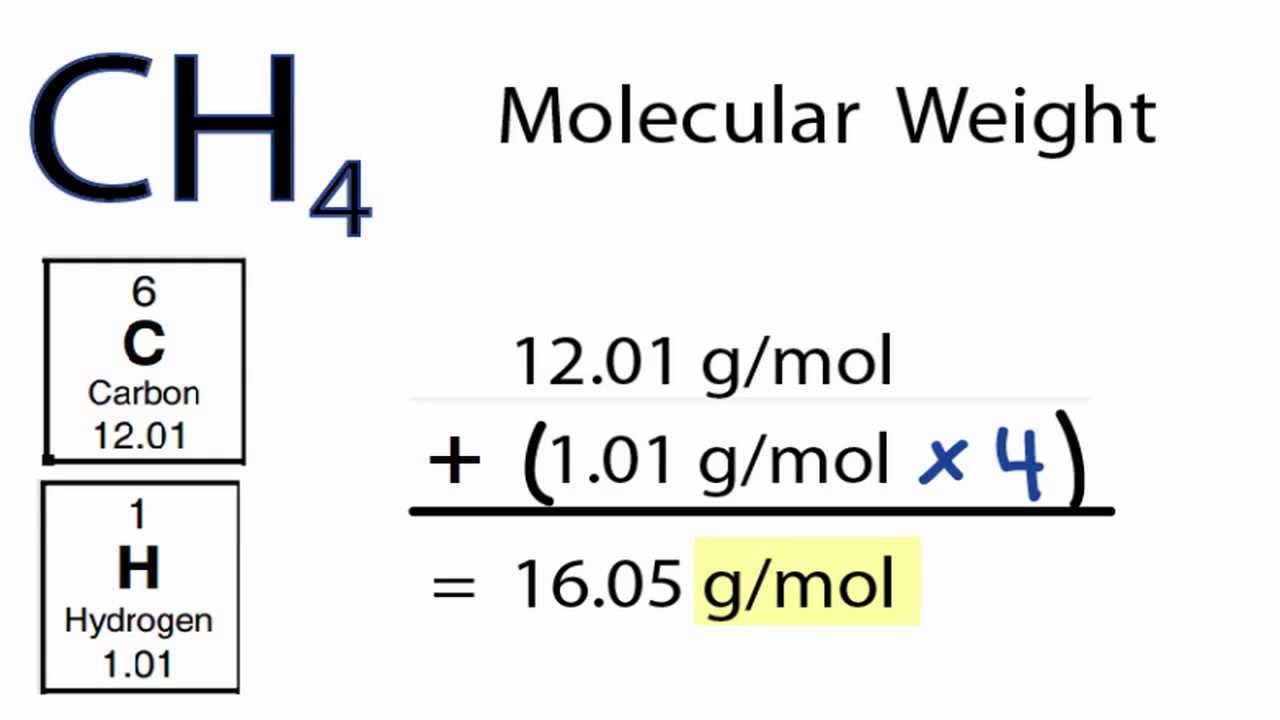
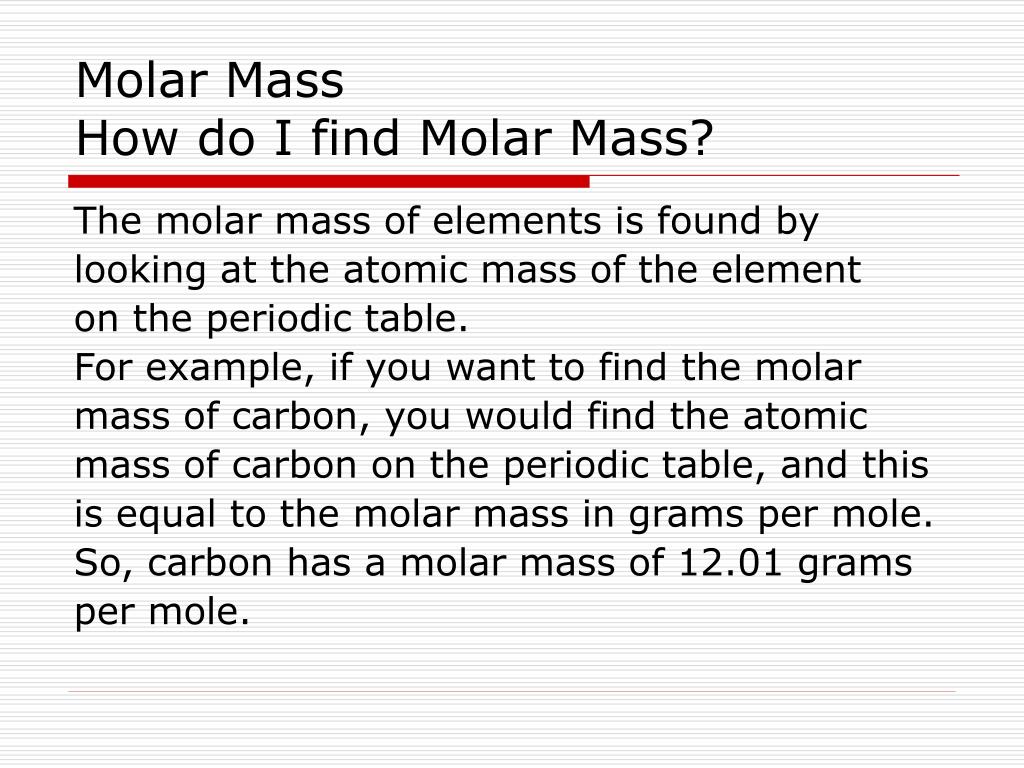
The link between the mass of an element and the number of atoms it contains is the relative atomic mass of the element. Find your atomic masses according to wikipedia an atomic mass unit unified is equal to 112 of a carbon 12 atom and is denoted with the letter u which stands for units. To calculate the molecular mass of a compound you need to know two things. By using this chemist work out the chemical formula.
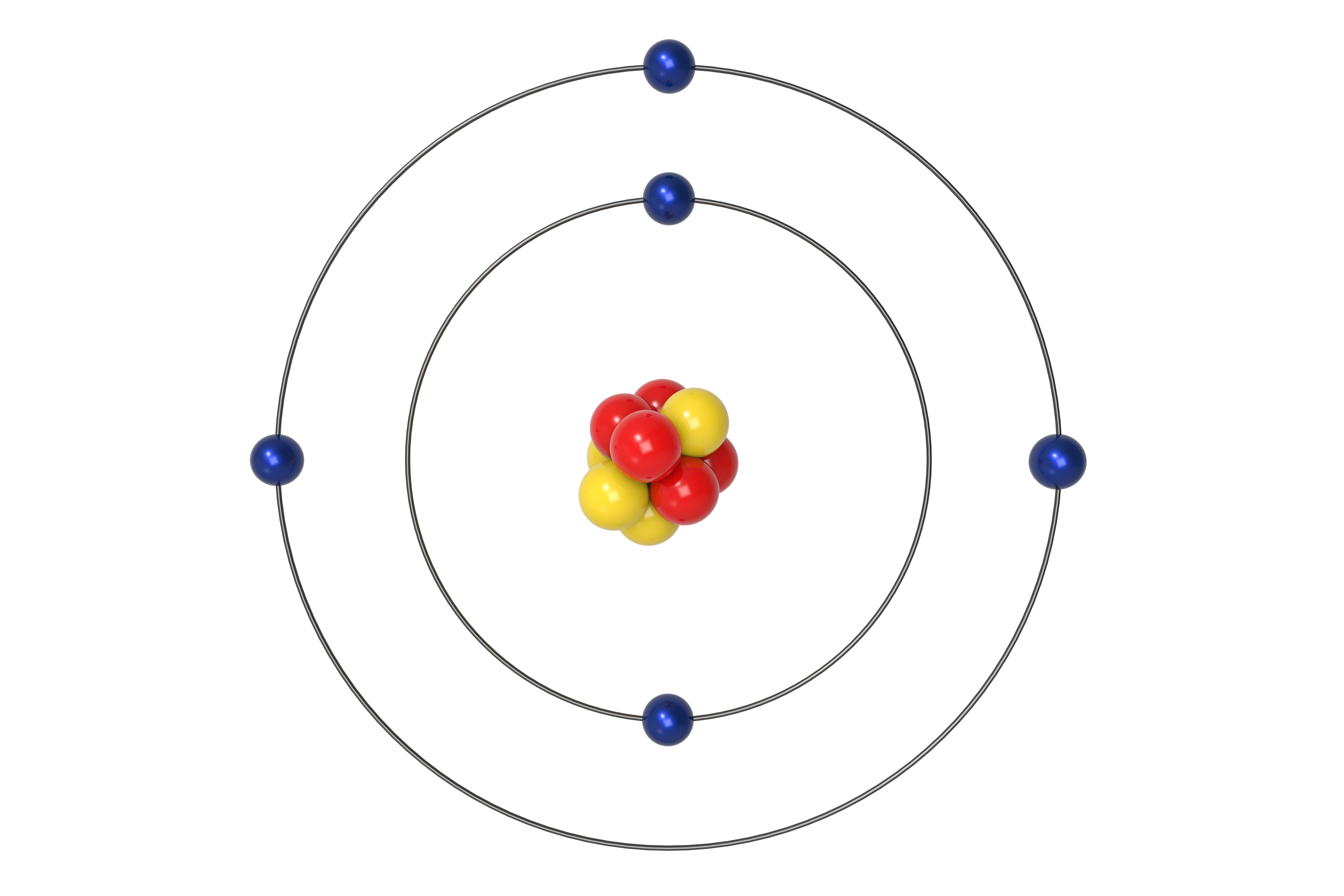
How to find the mass number atomic number and atomic mass number. The small contribution of mass from electrons is disregarded in calculating the mass number. The units for. The first is the molecular formula and the second is the atomic mass number of each of the elements that comprise it.
Find the molar mass of adrenaline. Finding the mass number. This approximation of mass can be used to easily calculate how many neutrons an element has by simply subtracting the number of protons from the mass number. Every element is characterized by a unique number of positively charged protons in.
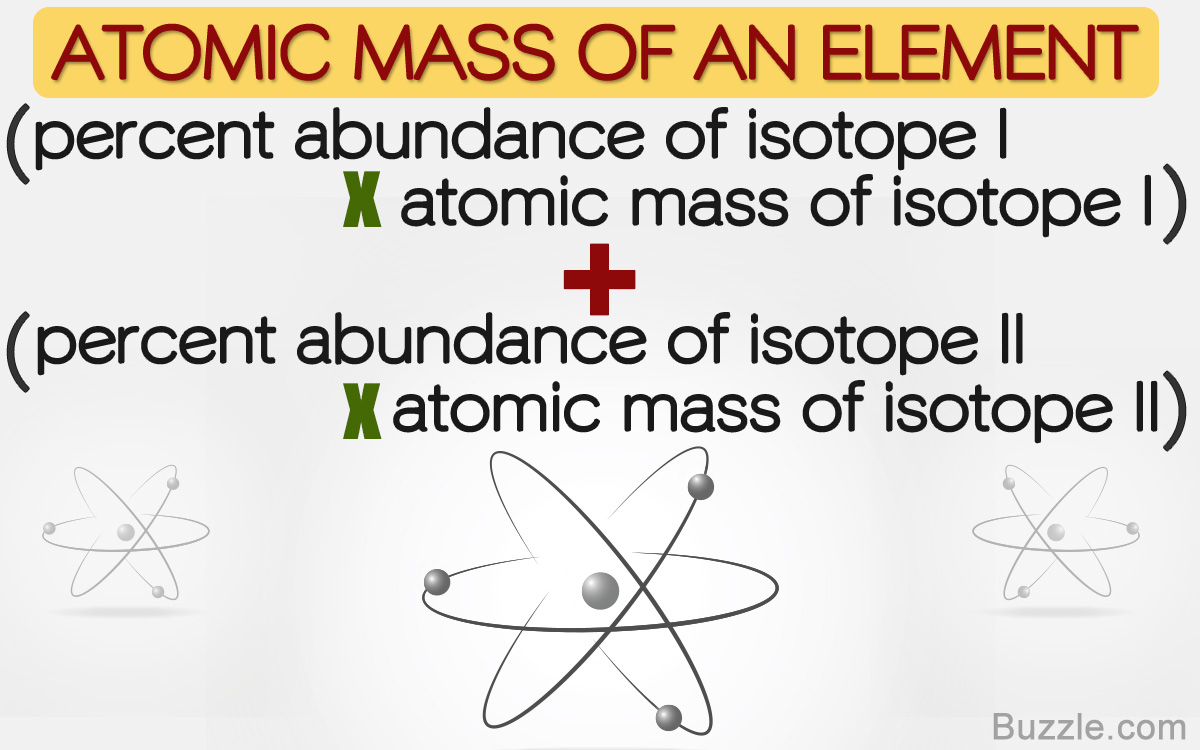
The average atomic mass of an element with n isotopes equals massisotope 1 abundanceisotope 1 massisotope 2 abundanceisotope 2.


/atomic-weight-and-atomic-mass-difference-4046144_FINAL_STILL-5940e35000b145ba83fb8e3e40792ba9.png)























:max_bytes(150000):strip_icc()/GettyImages-175532236-c614b233b7e84d5487cad8b280f365a4.jpg)



/mass-percent-composition-example-609567_V2-01-89c18a9d30ea43b494d09b81f7ffefc1.png)









/atomic-mass--58dc0d885f9b58468332c41b.jpg)