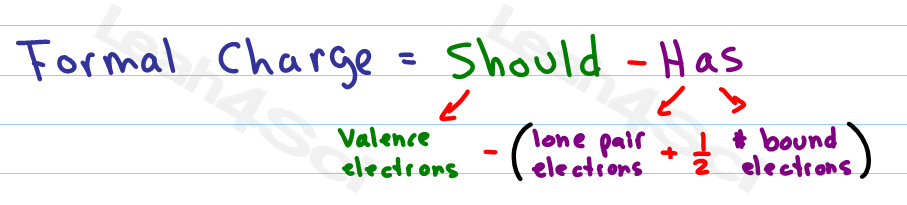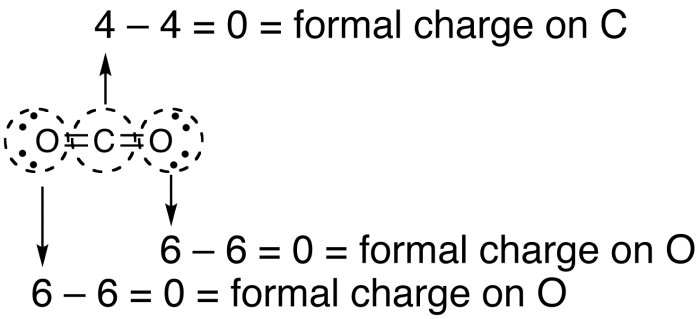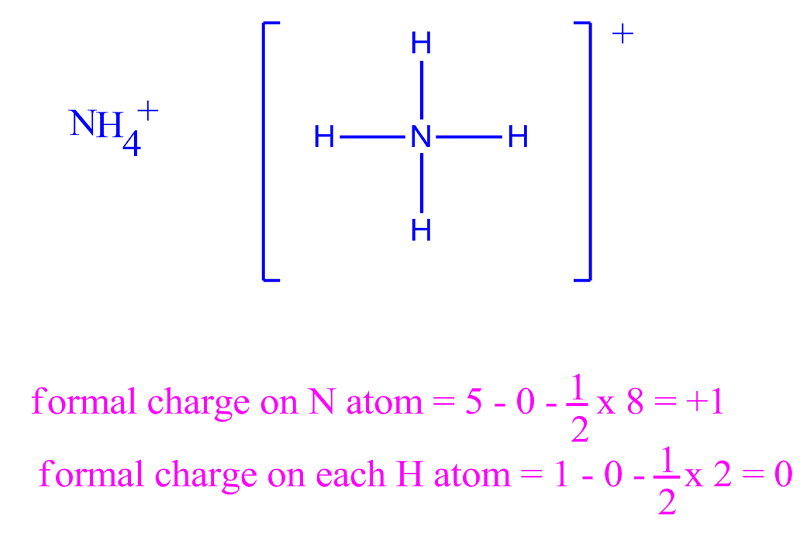How To Calculate Formal Charge
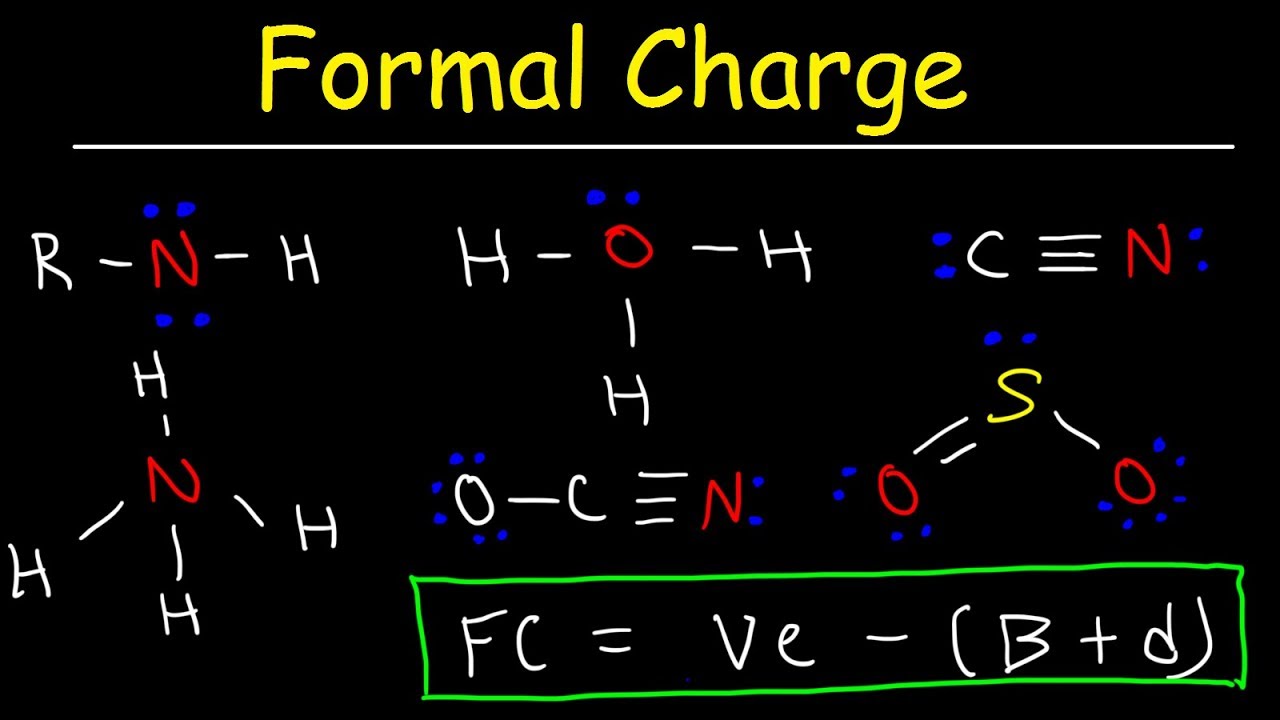
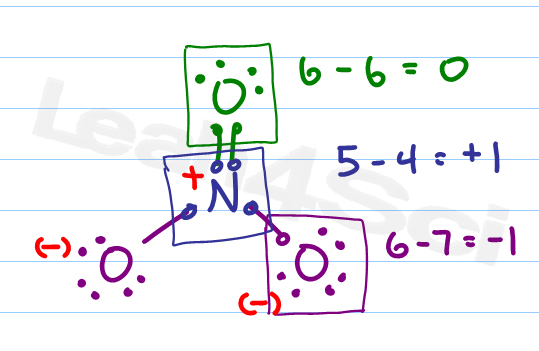
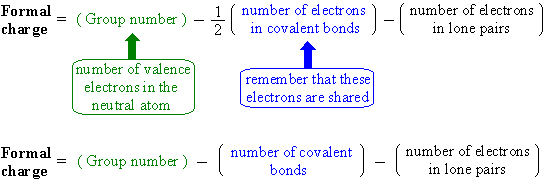
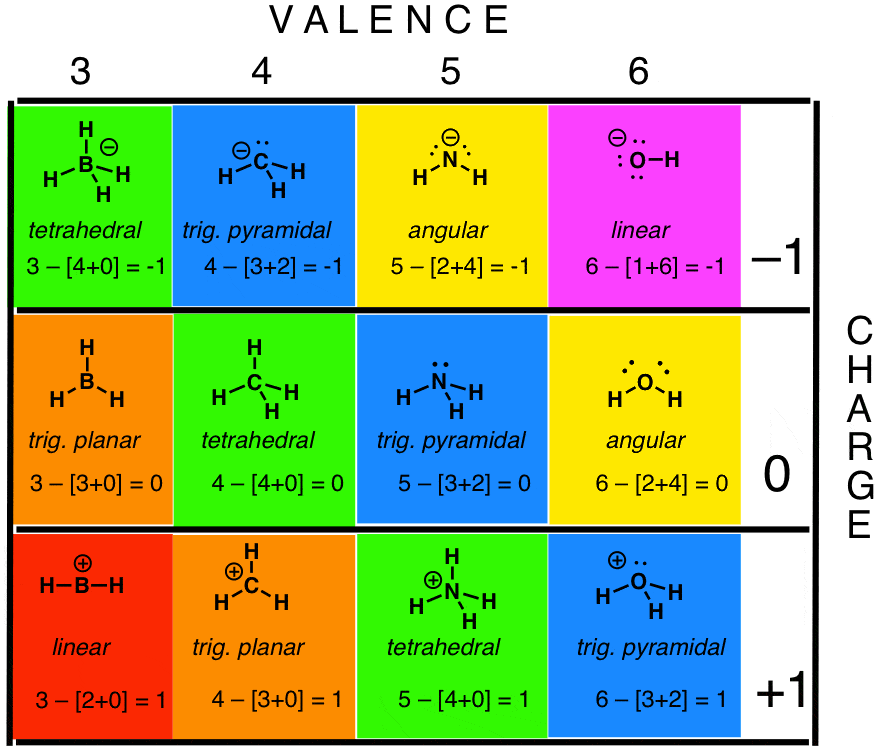
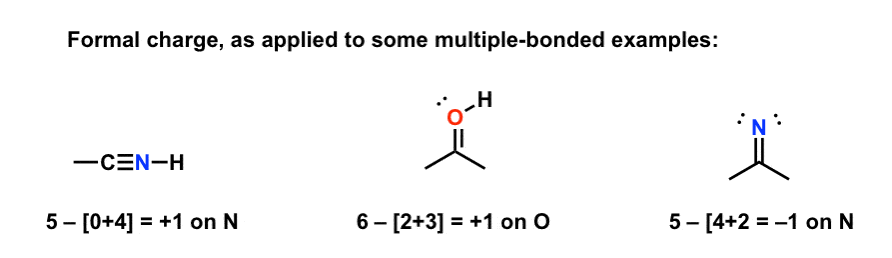
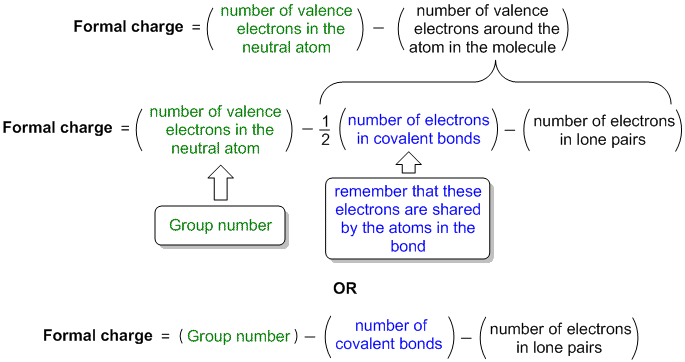
Formal charge equation formal charge valence electrons on neutral atom lone electron pairs bonding electrons valence electrons corresponds to the group number of the periodic table for representative elements.

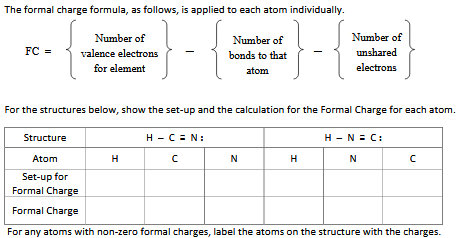
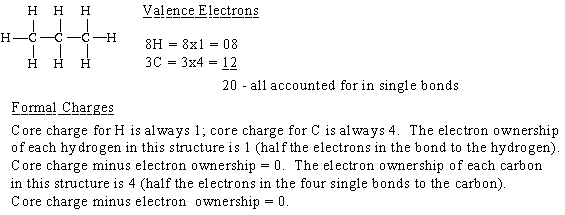
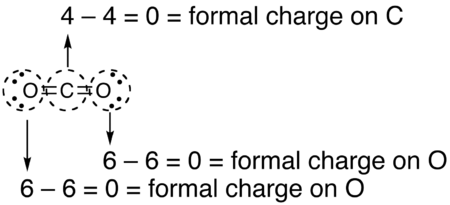
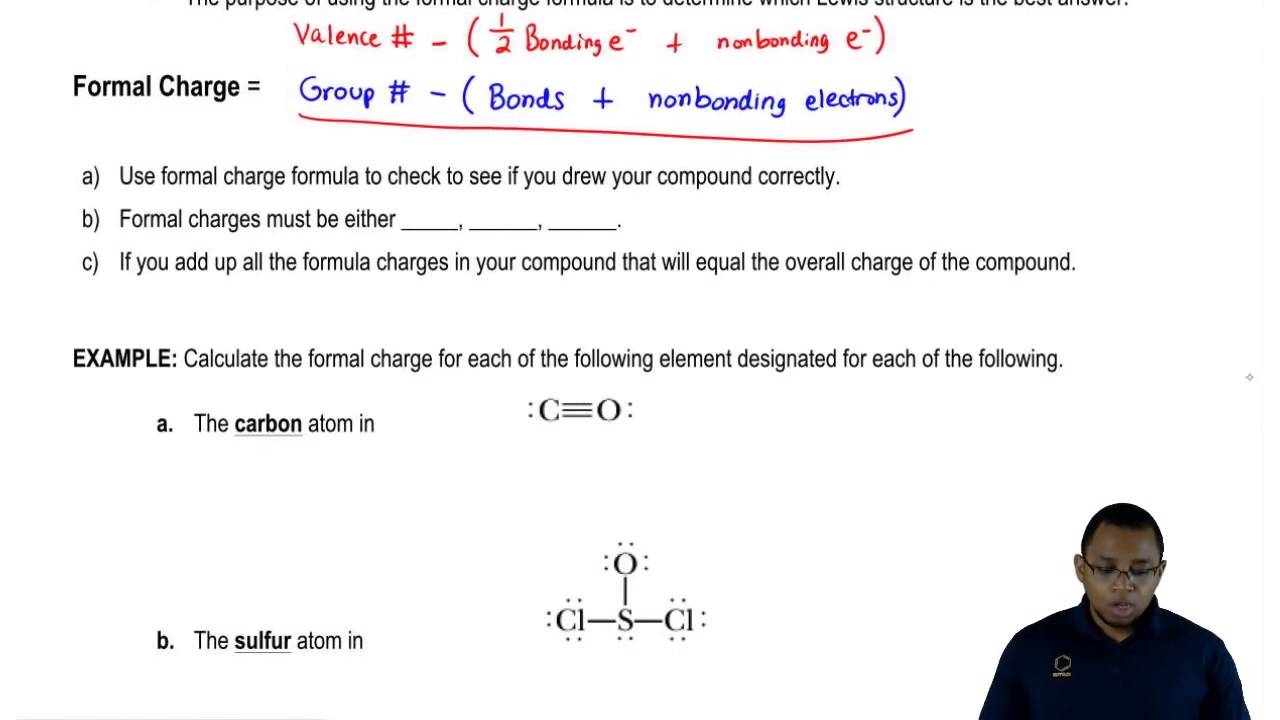
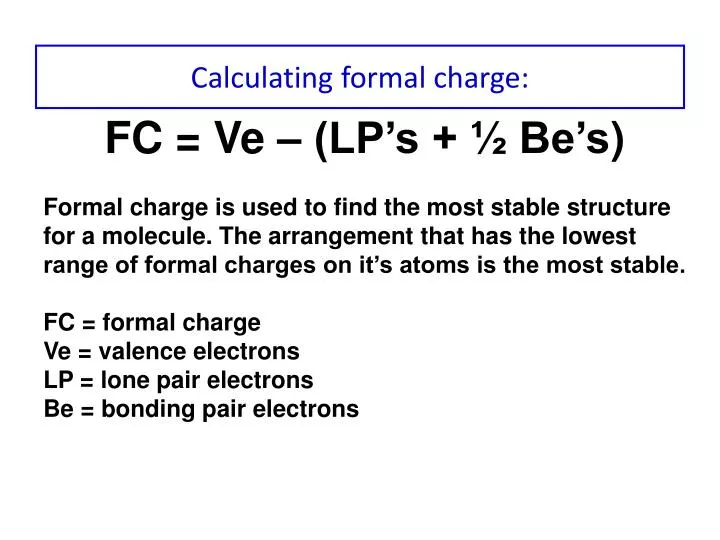
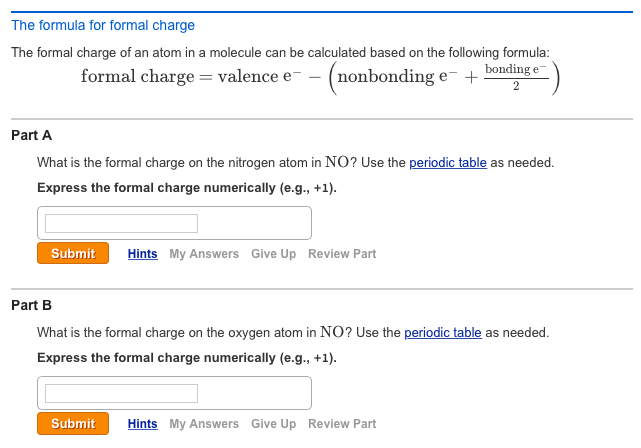
How to calculate formal charge. The formal charge of an atom can be determined by the following formula. The formal charge of any atom in a molecule can be calculated by the following equation. Identifying a formal charge involves. Formal charge 4 0 82 0.
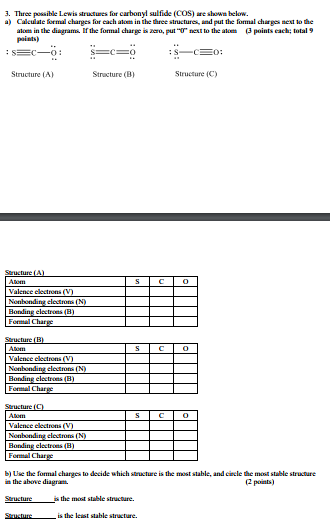
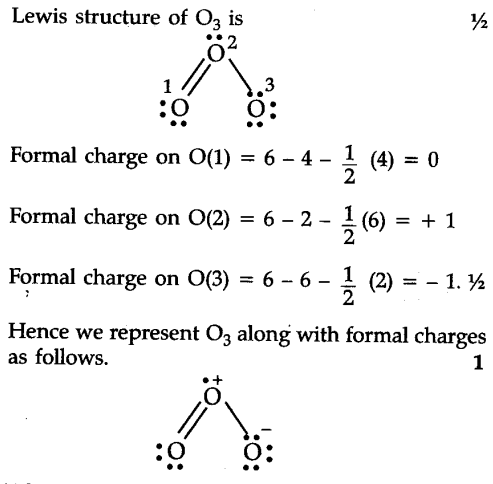
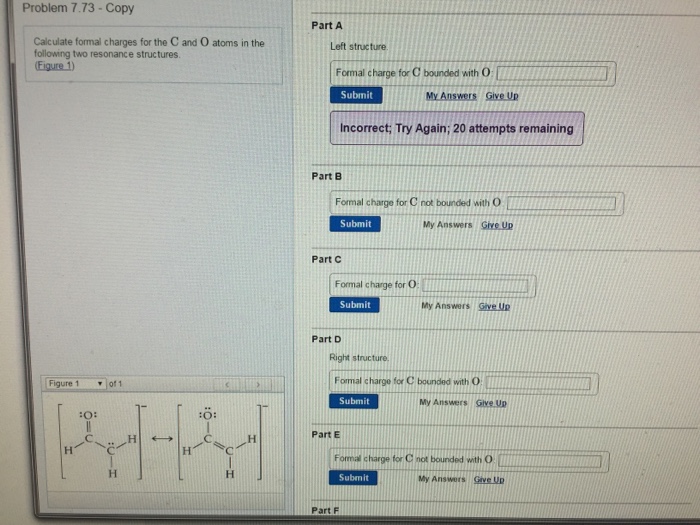
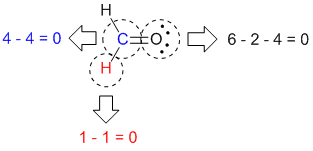
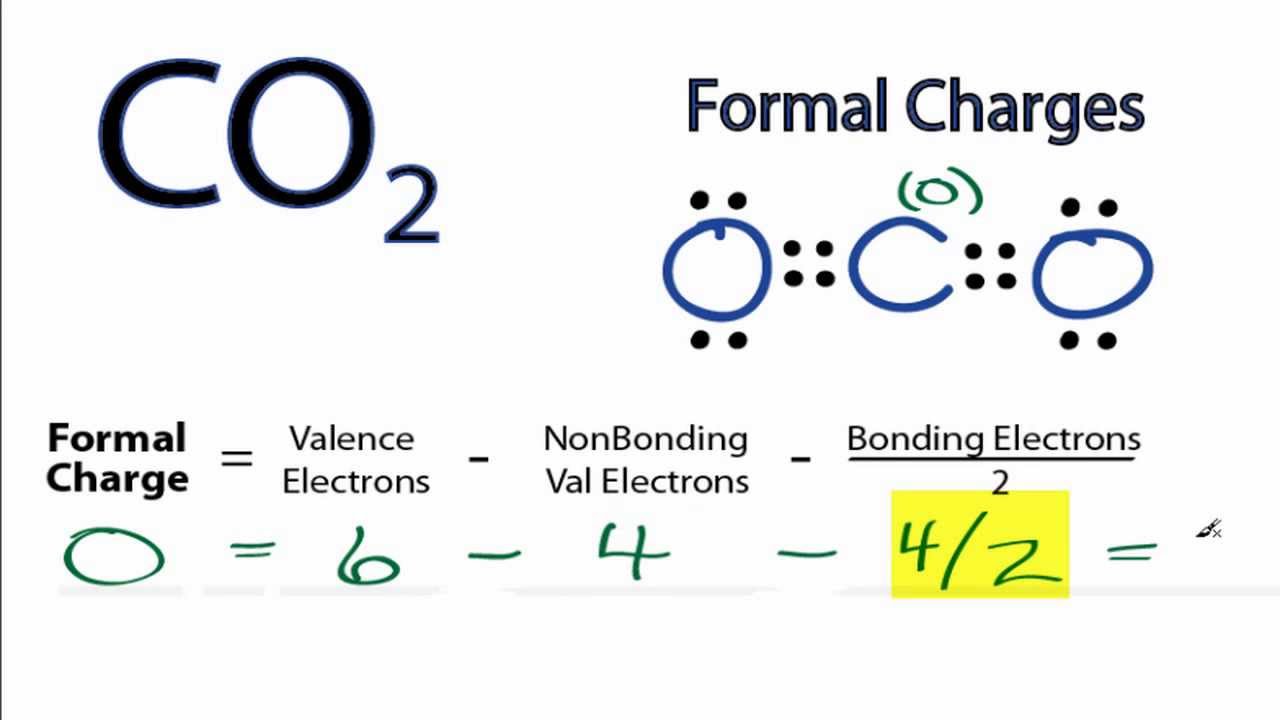
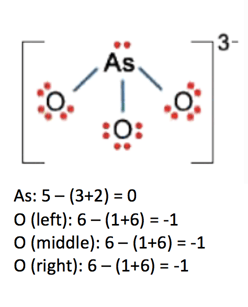
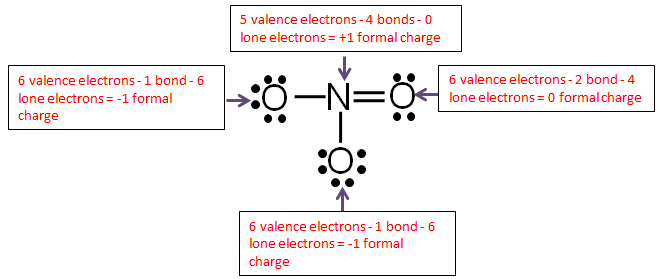
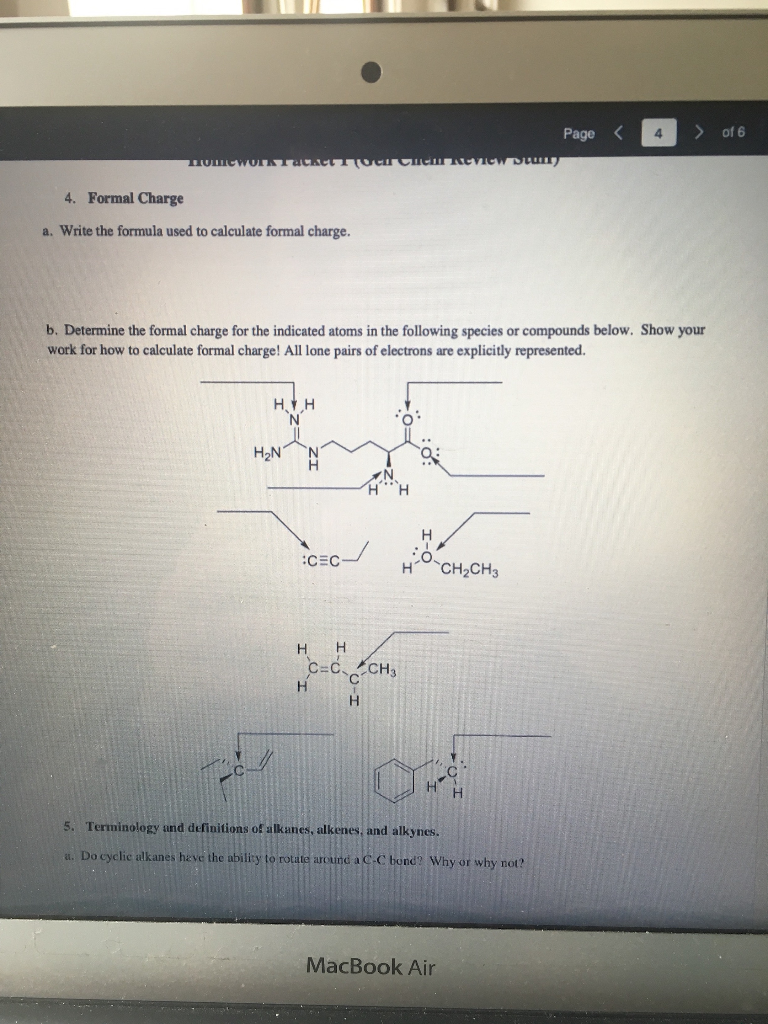
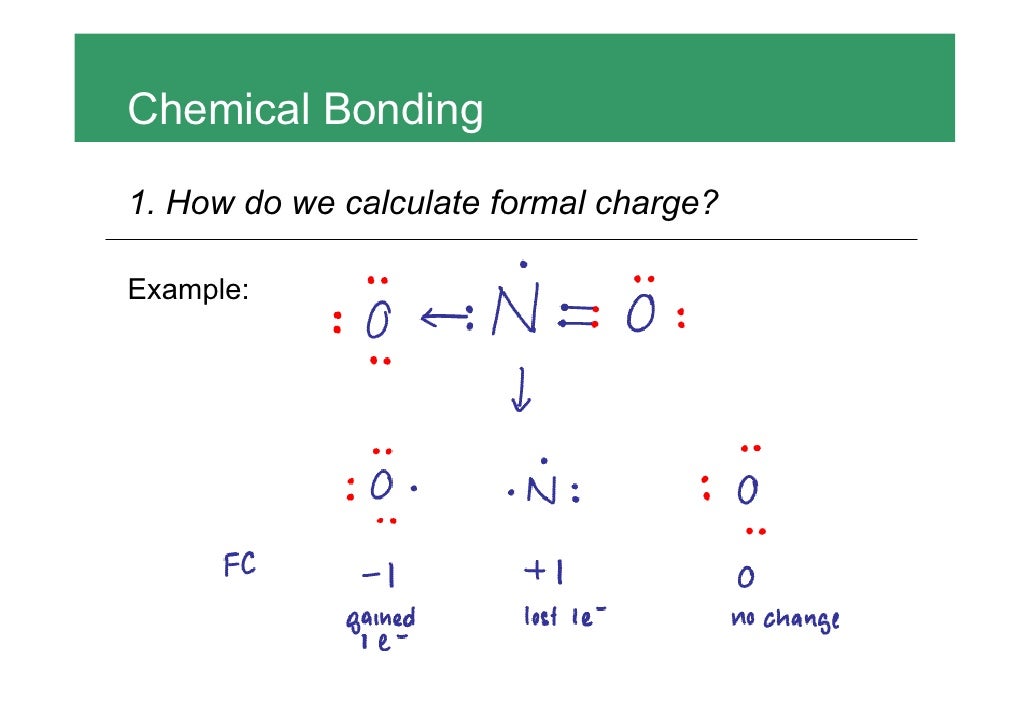
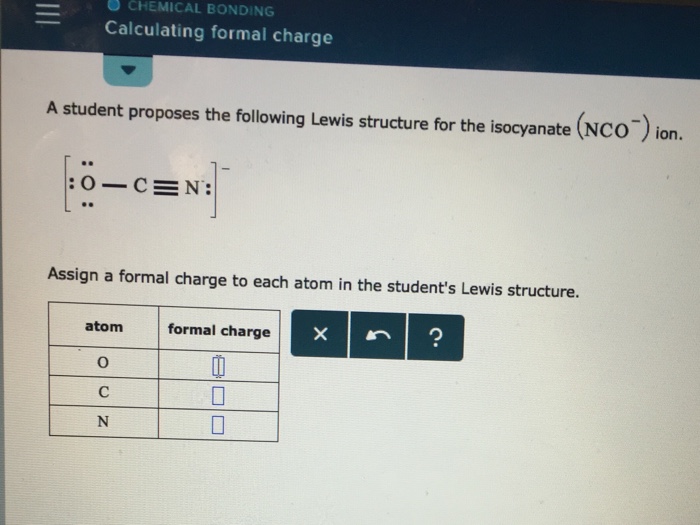
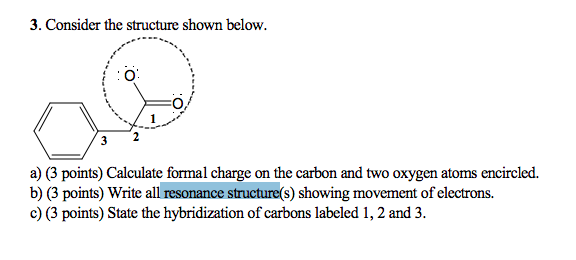
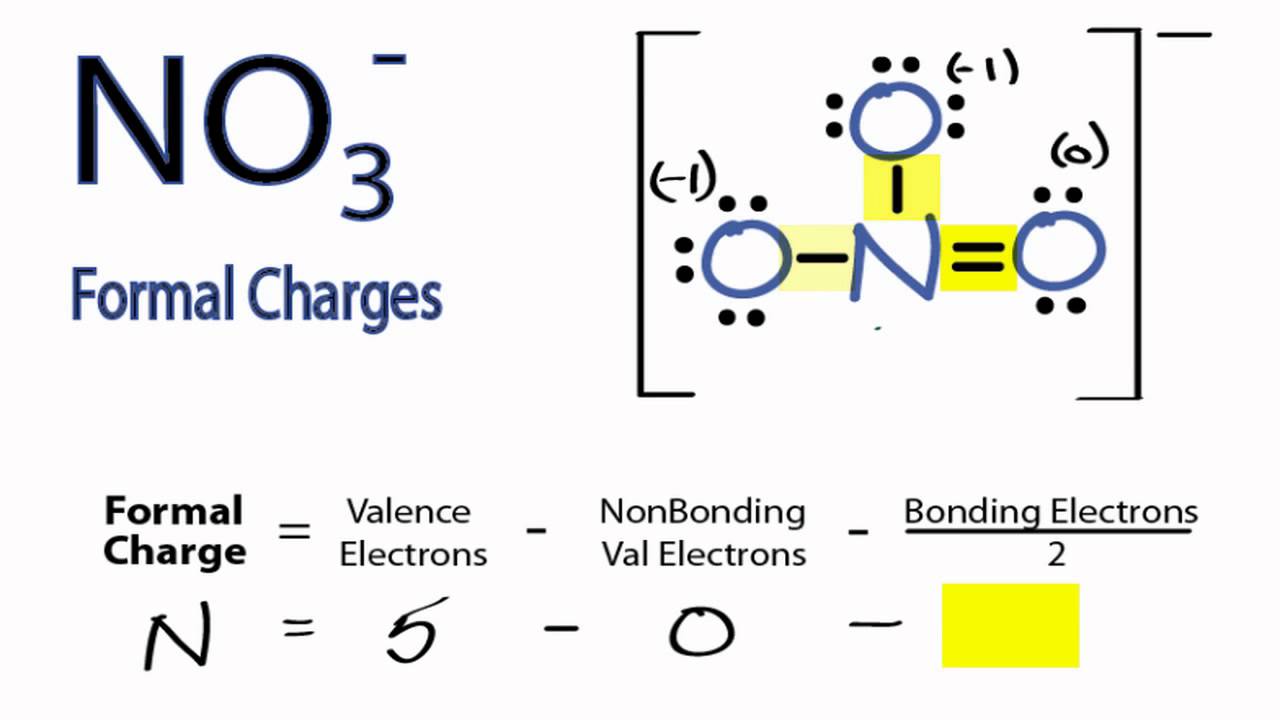
Calculate the formal charge of oxygen on the left. Determining whether the atom exhibits the appropriate number of electrons in the lewis structure determine whether. The formal charge of carbon is 0. A step by step description on how to calculate formal charges.
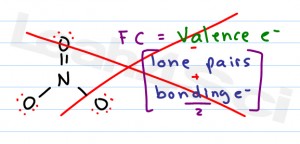
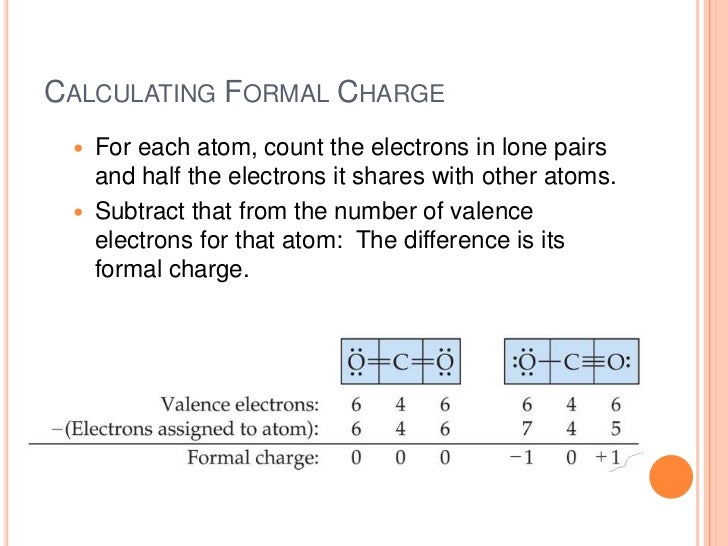
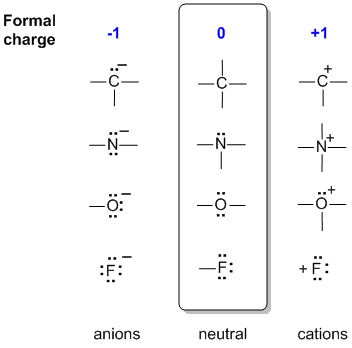
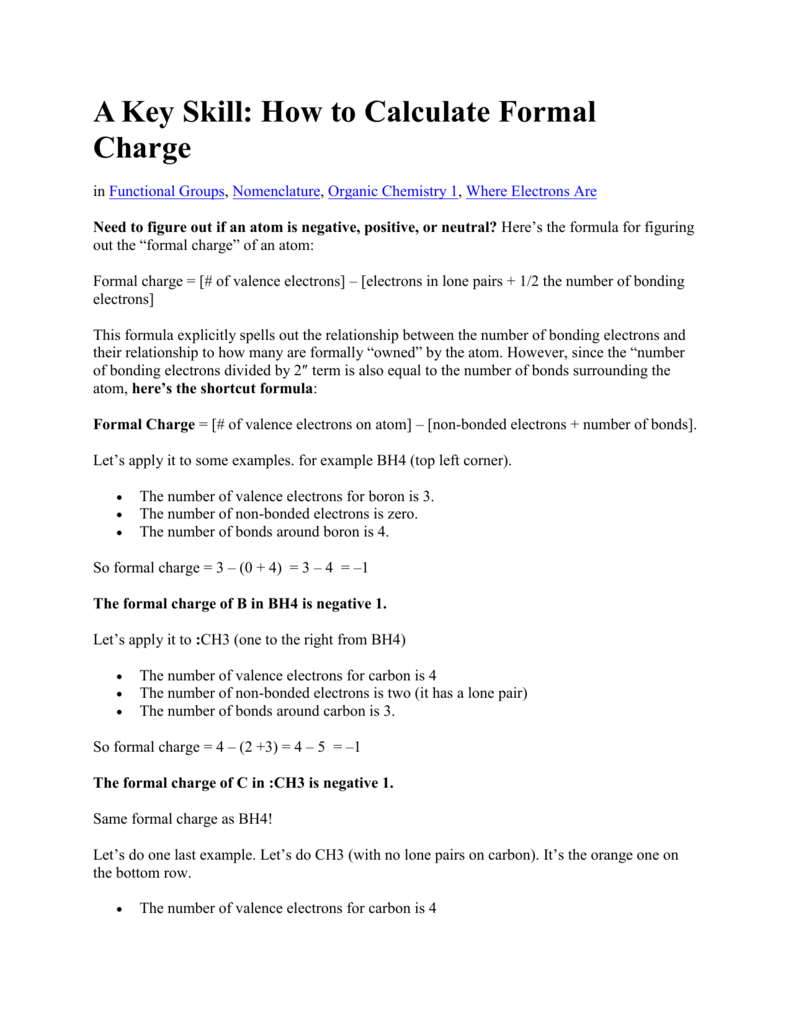
Formal charges are important because they allow us to predict which lewis structure is the most likely to exist in the real world. If it is a neutral molecule then the sum of all the formal charges must equal zero. Latexfc v n frac b 2 latex in this formula v represents the number of valence electrons of the atom in isolation n is the number of non bonding valence electrons and b is the total number of electrons in covalent bonds with other atoms in the molecule. Formal charge of valence electrons electrons in lone pairs 12 the number of bonding electrons this formula explicitly spells out the relationship between the number of bonding electrons and their relationship to how many are formally owned by the atom.
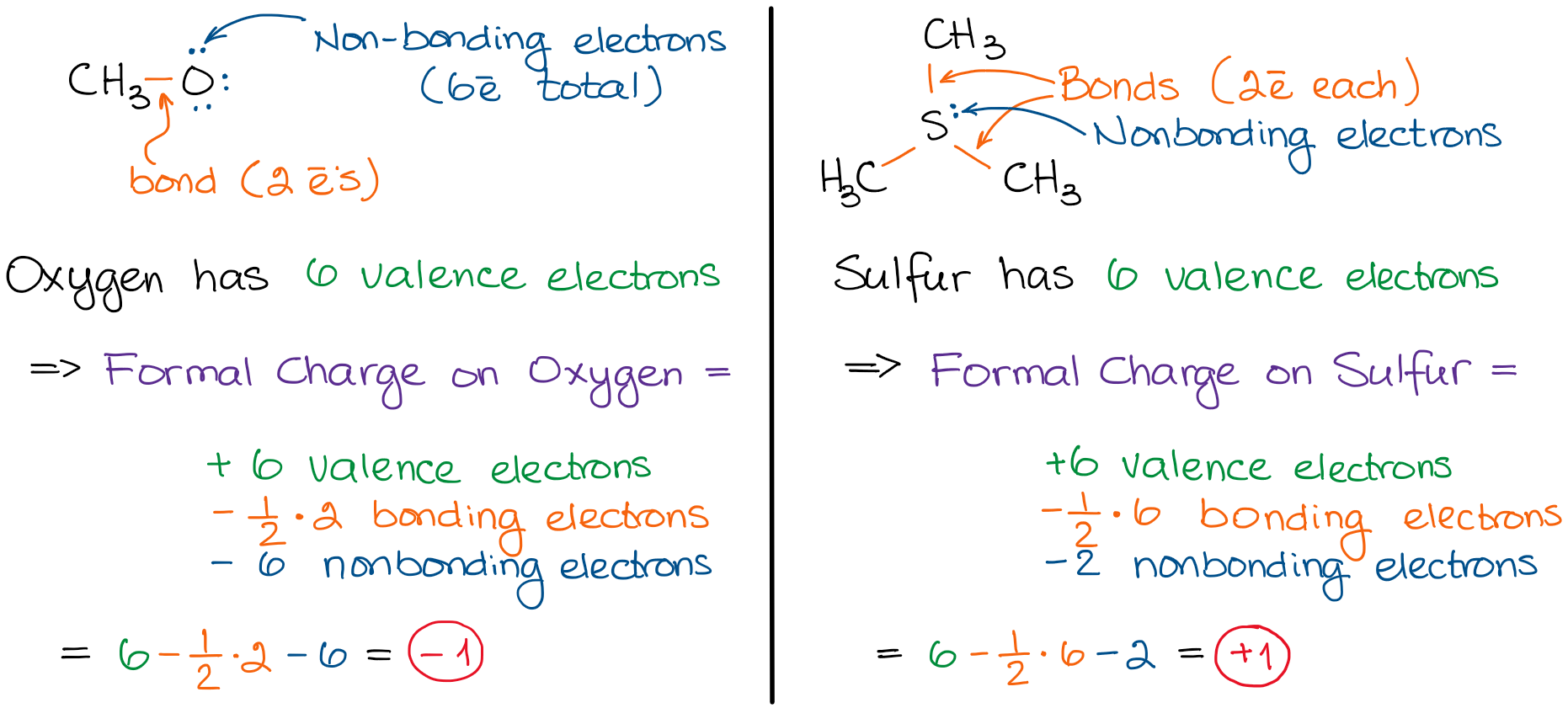
N is the number of non bonding valence electrons on this atom in the molecule. How to calculate formal charge once we add all the formal charges for the atoms in the lewis structure we should get a value equal to the actual charge of the molecule or ion. Determining the appropriate number of valence electrons for an atom this can be accomplished by inspecting the. F c v n b 2 displaystyle fcv n frac b2 where v is the number of valence electrons of the neutral atom in isolation in its ground state.
And b is the total number of electrons shared in bonds with other atoms in the molecule.

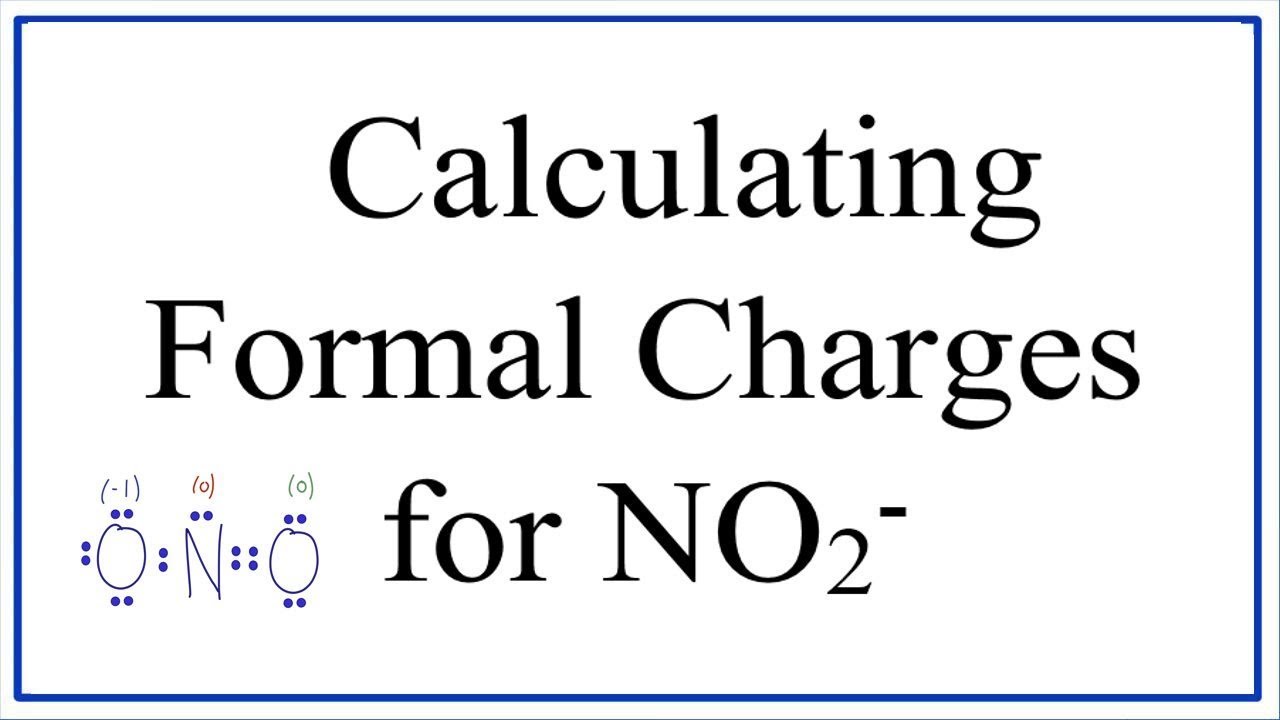










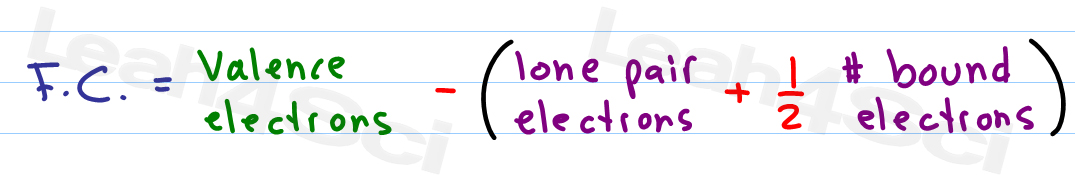


:max_bytes(150000):strip_icc()/1470px-H2O_Lewis_Structure-c55fbfc92823400097054565706aeff2.jpg)