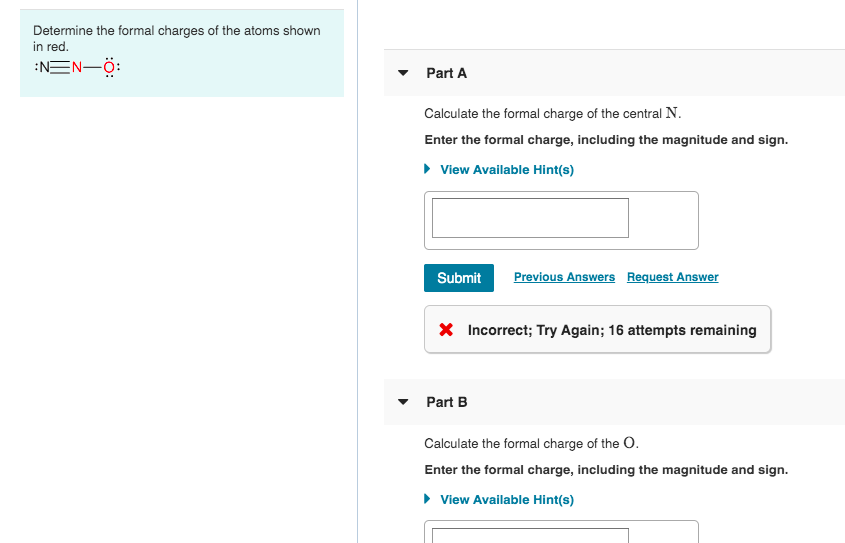
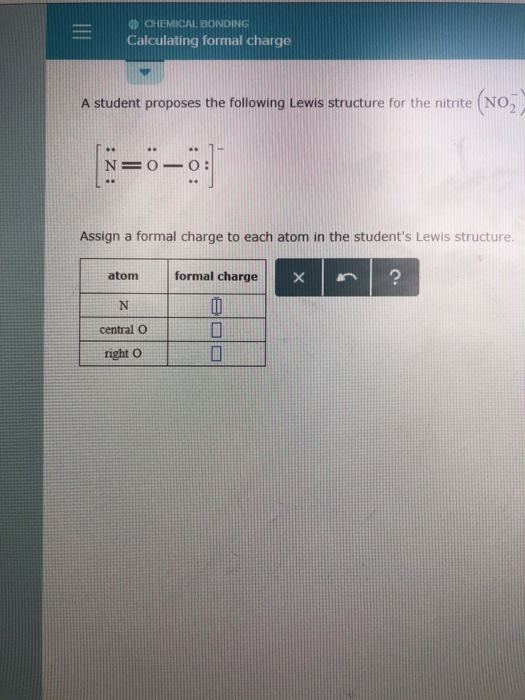
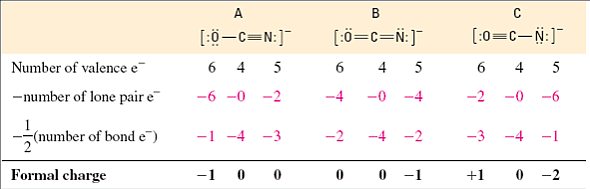
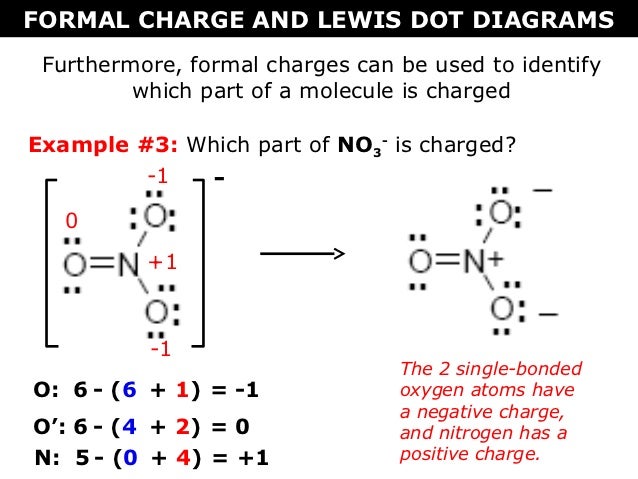
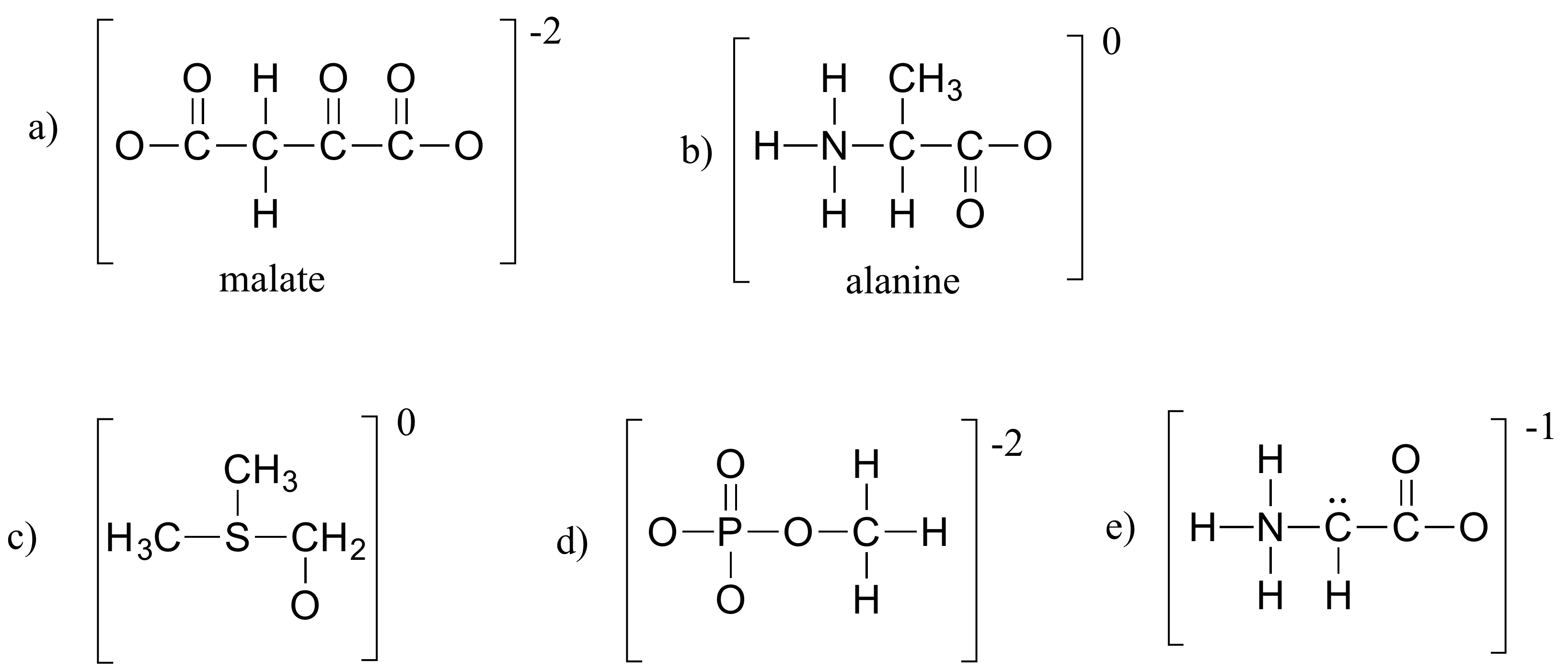
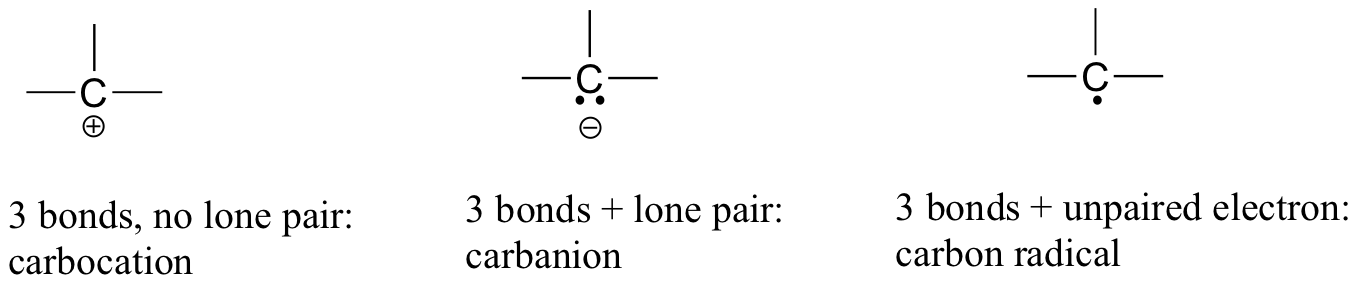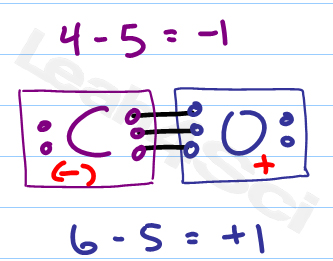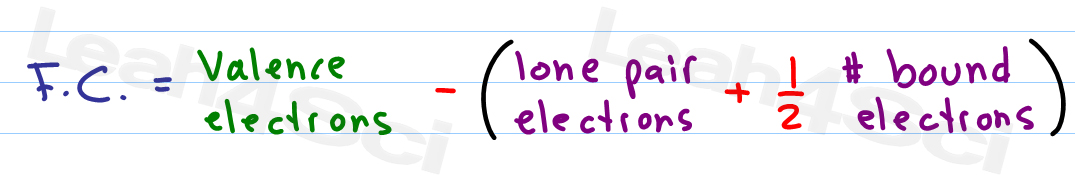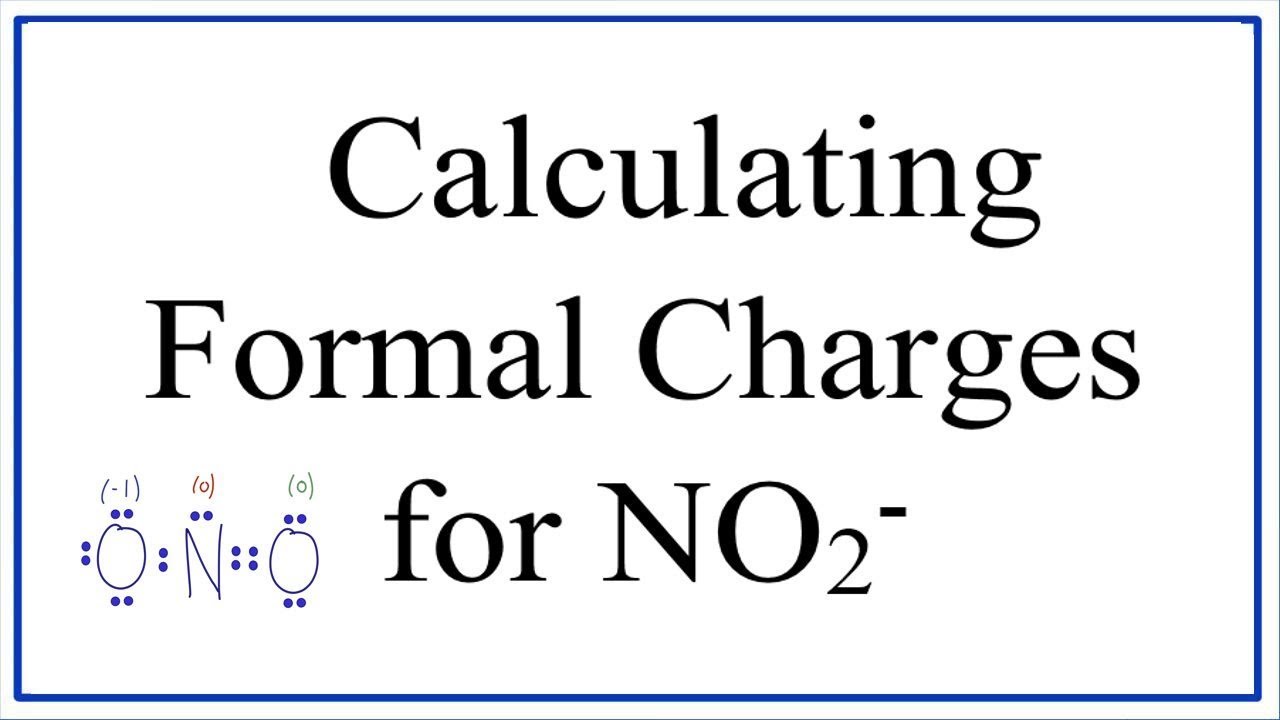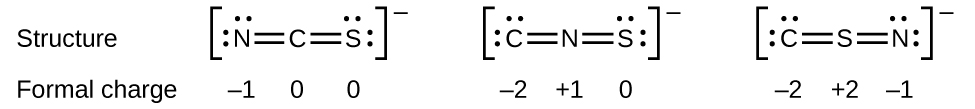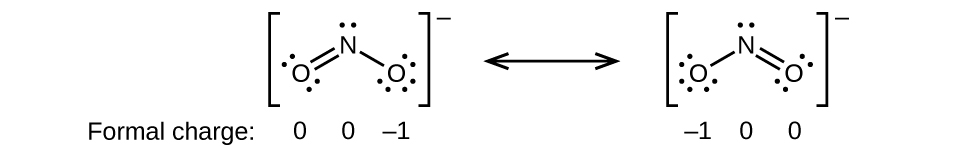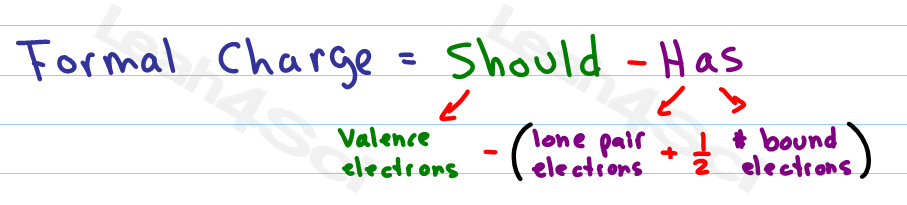How To Calculate Formal Charge Of Central Atom
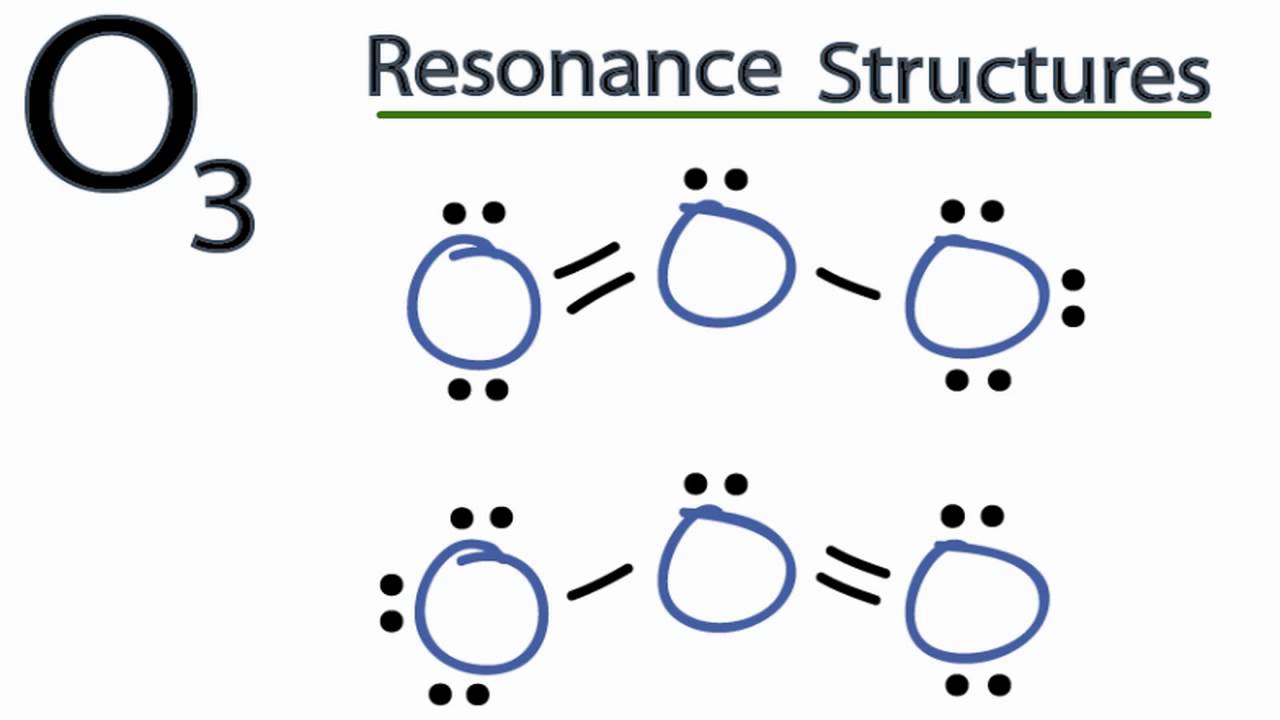
One line corresponds to two electrons.
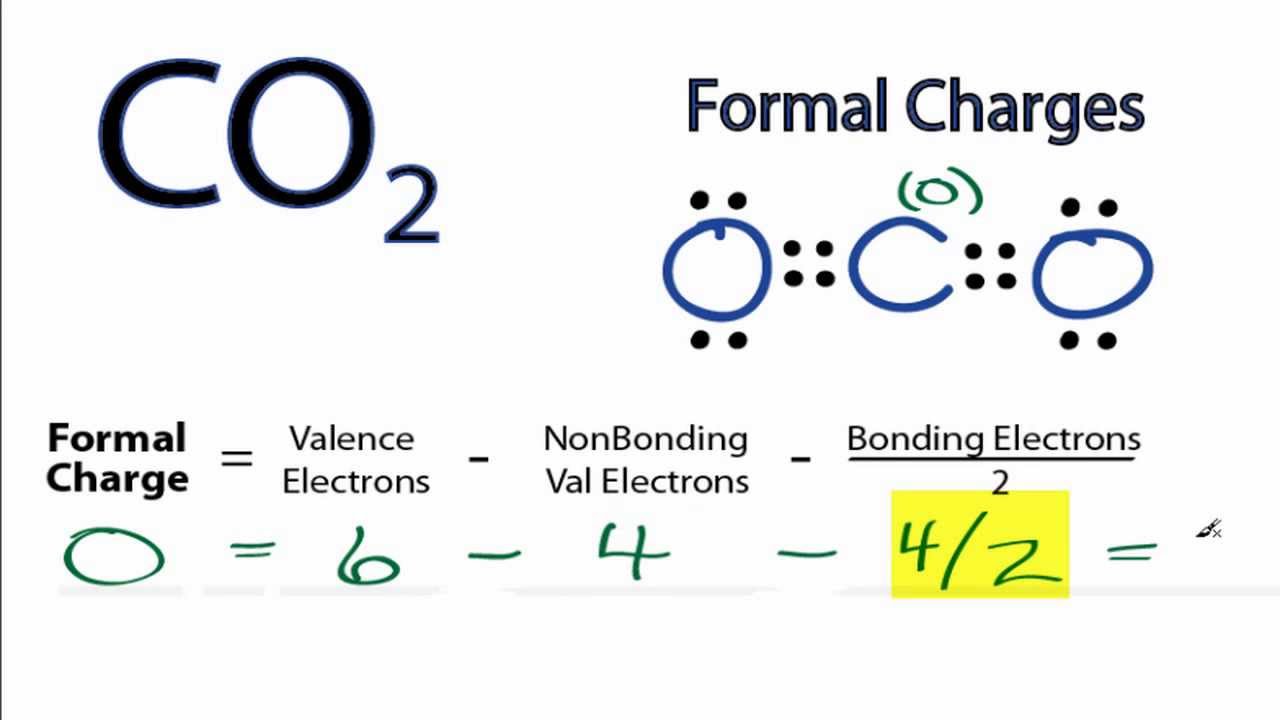
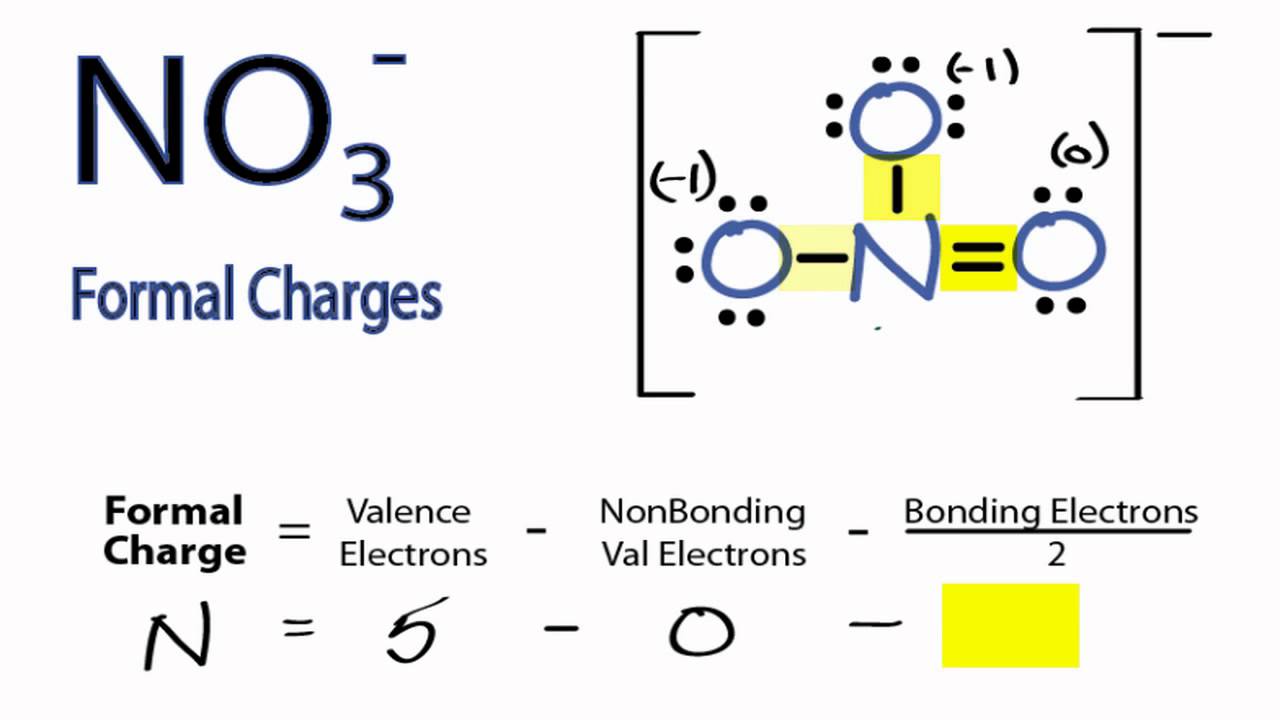
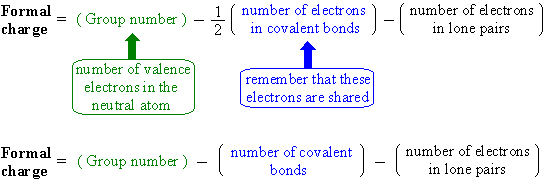
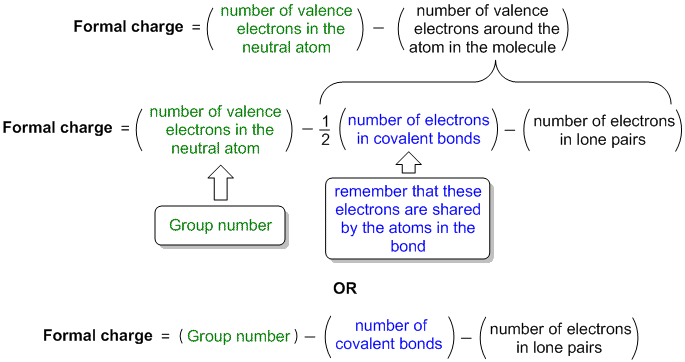
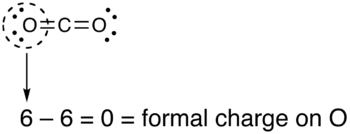
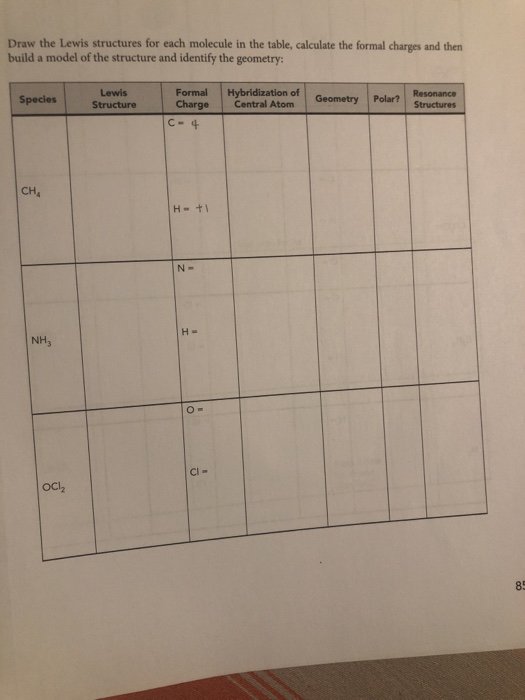
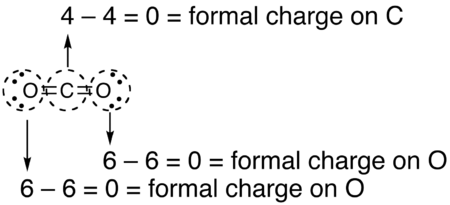
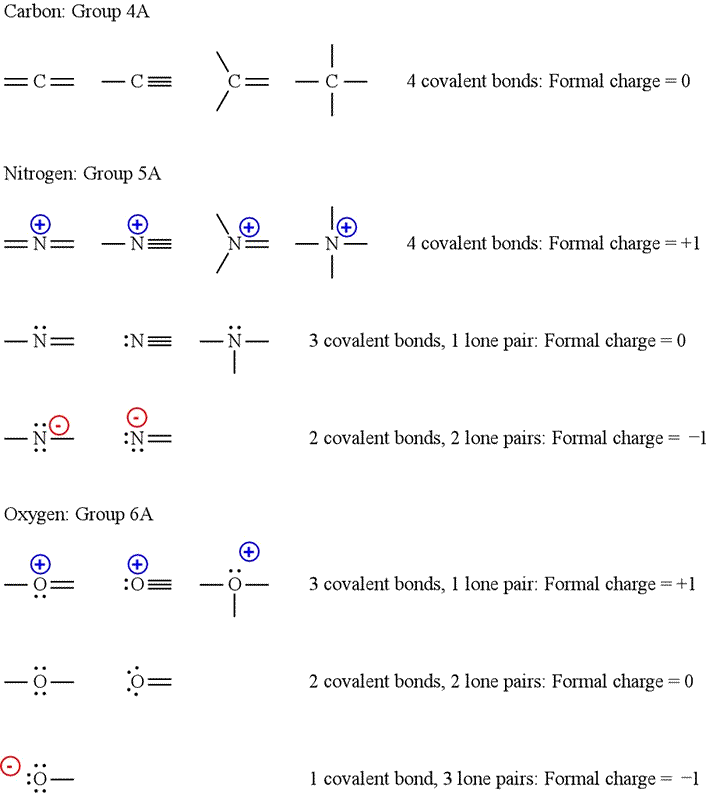
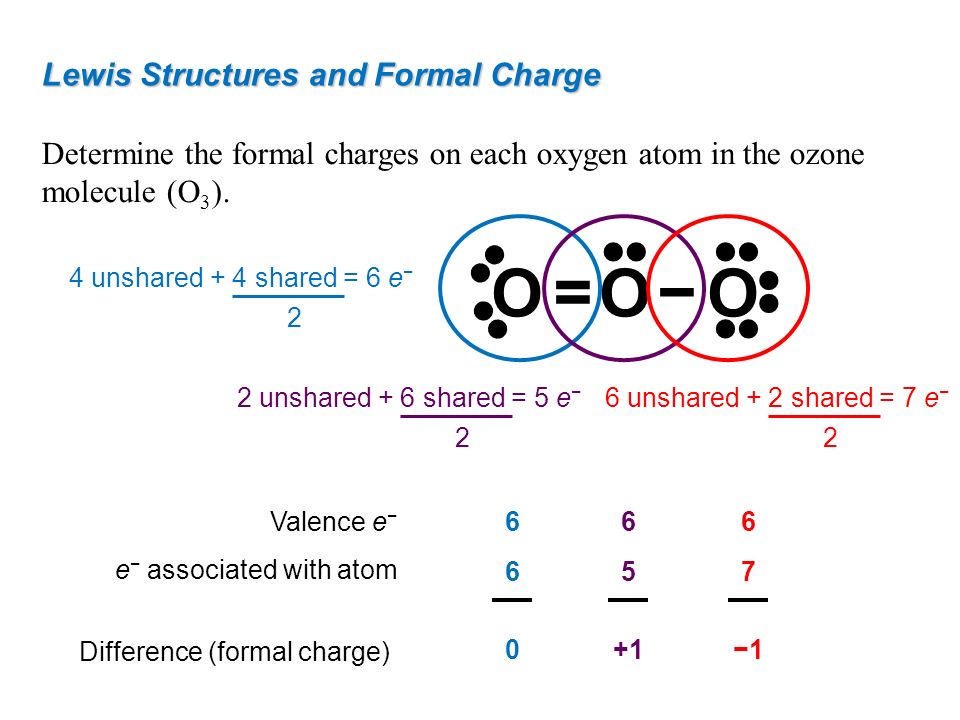
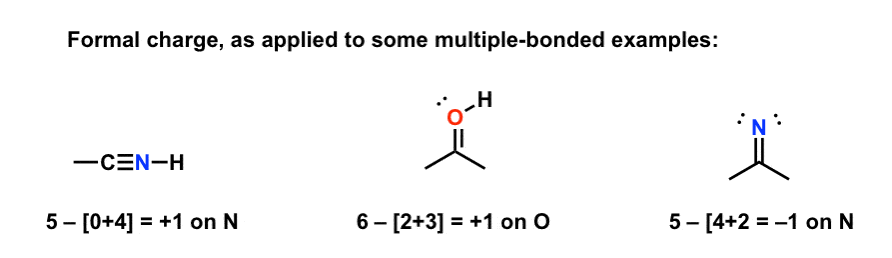
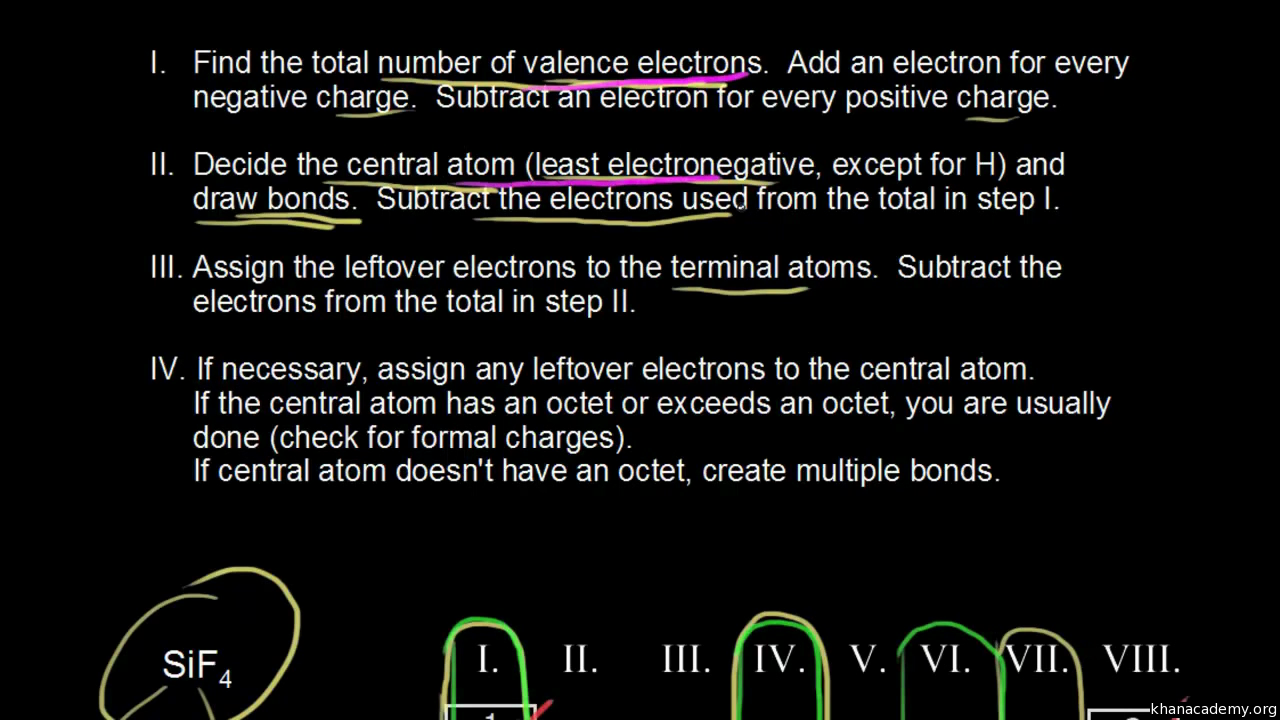
How to calculate formal charge of central atom. Formal charge no of valence electrons in central atom total no. Total number of unshared electrons 0. The formal charge of an atom can be determined by the following formula. Total number of shared electrons 8.
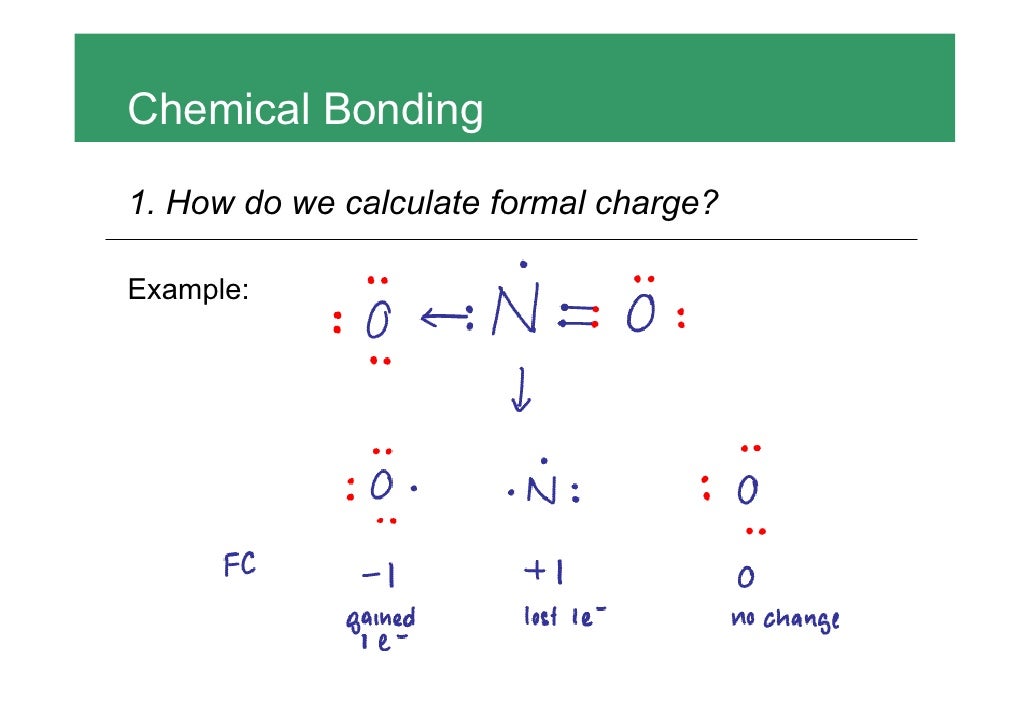
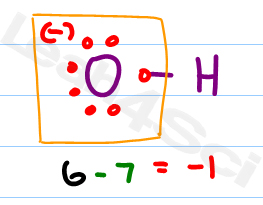
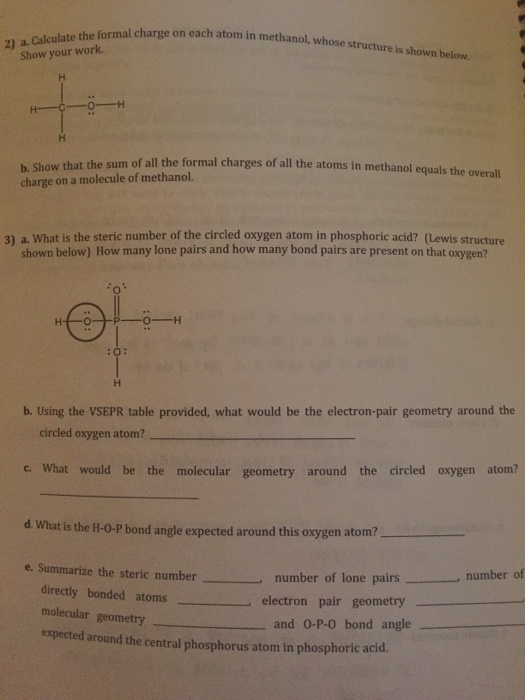
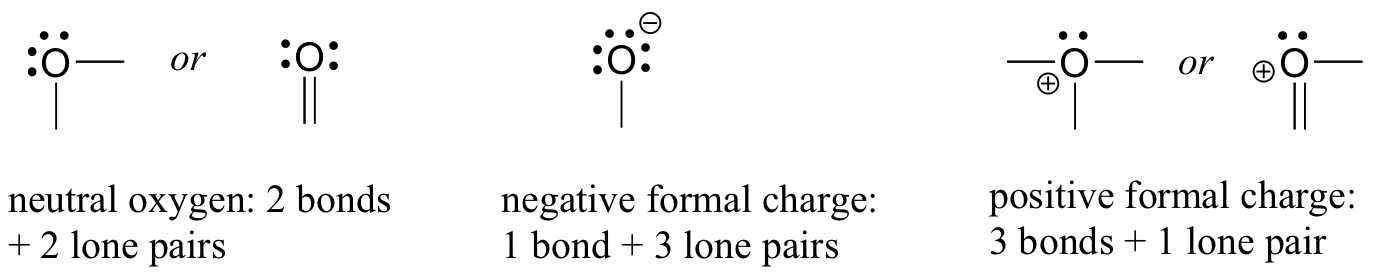
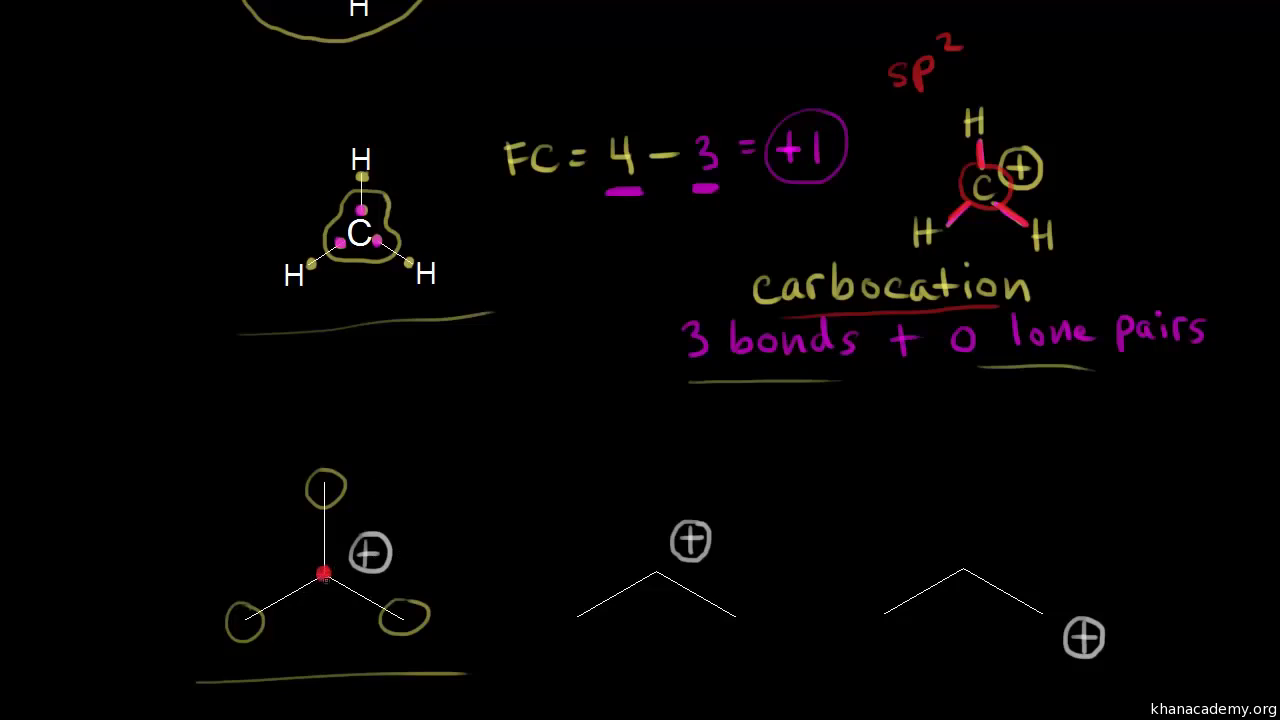
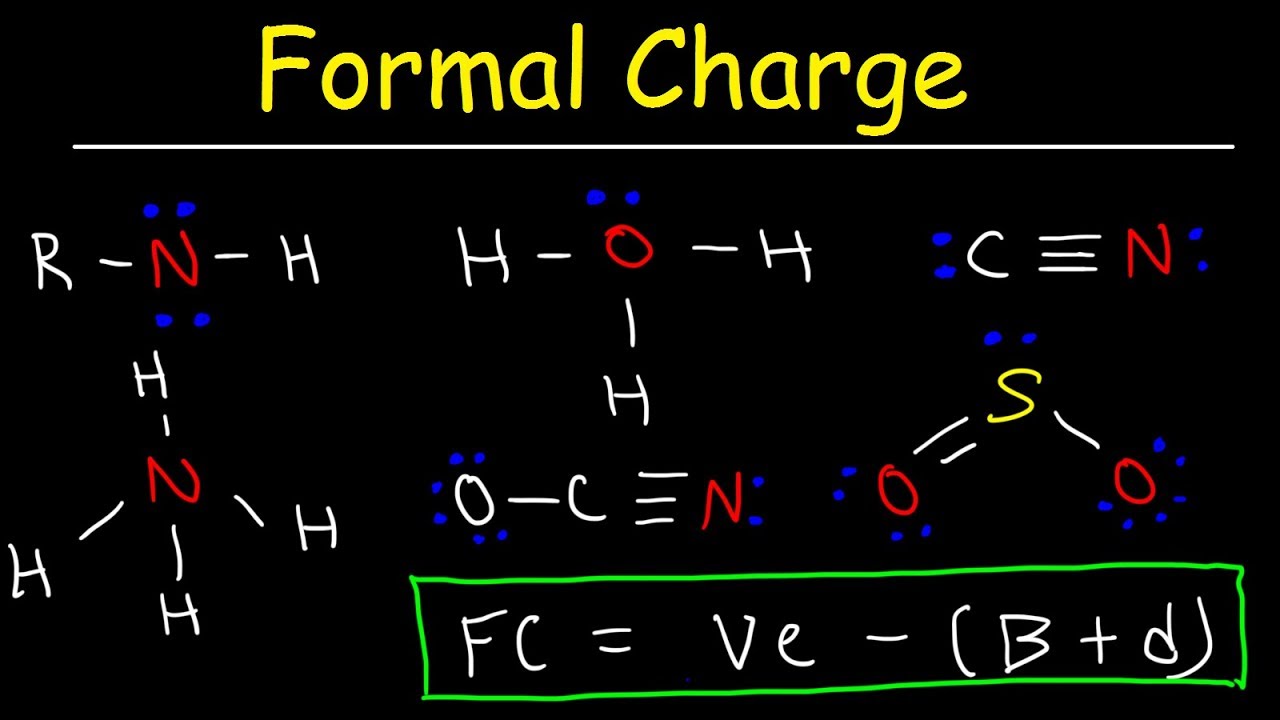
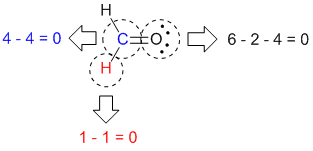
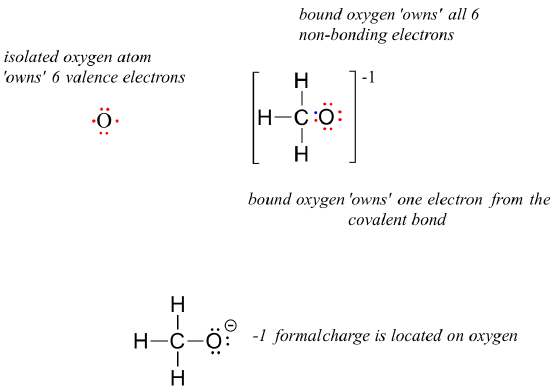
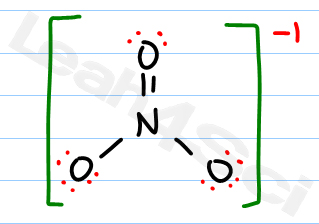
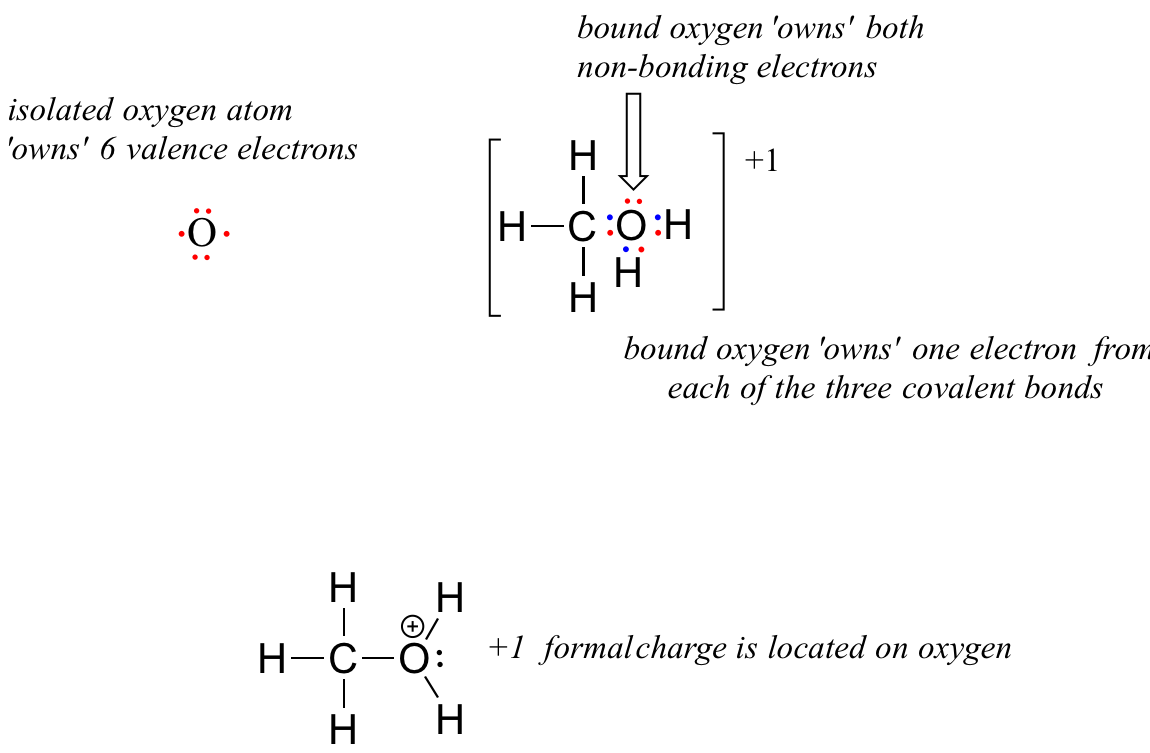
O 4 electrons from lone pairs plus 2 electrons from bonds 6 electrons. Each electron counts as one and so a pair counts as two. The result is the formal charge for that atom. In this example the nitrogen and each hydrogen has a formal charge of zero.
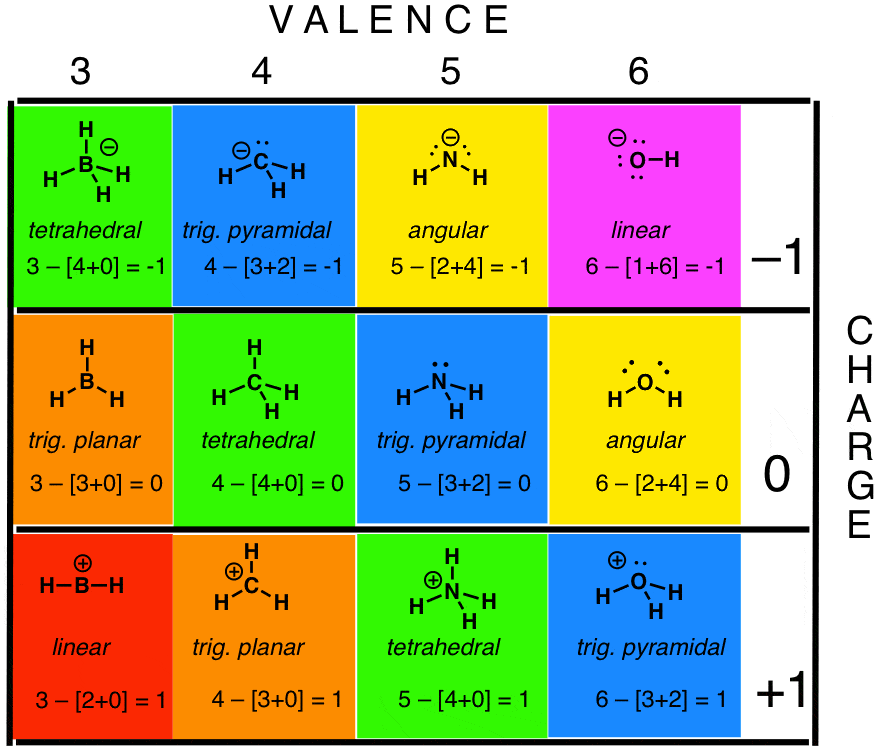
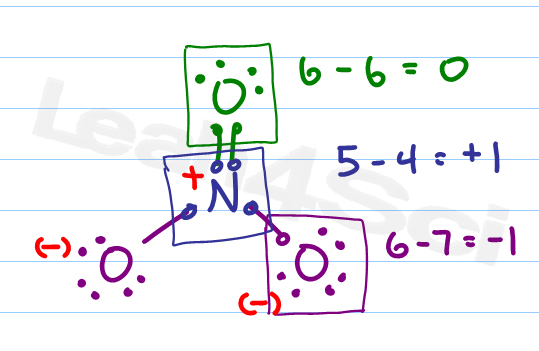
Latexfc v n frac b 2 latex in this formula v represents the number of valence electrons of the atom in isolation n is the number of non bonding valence electrons and b is the total number of electrons in covalent bonds with other atoms in the molecule. If it is a neutral molecule then the sum of all the formal charges must equal zero. Cl 6 electrons from lone pairs plus 1 electron from a bond with c 7 electrons. Valence electrons in free p atom 5.
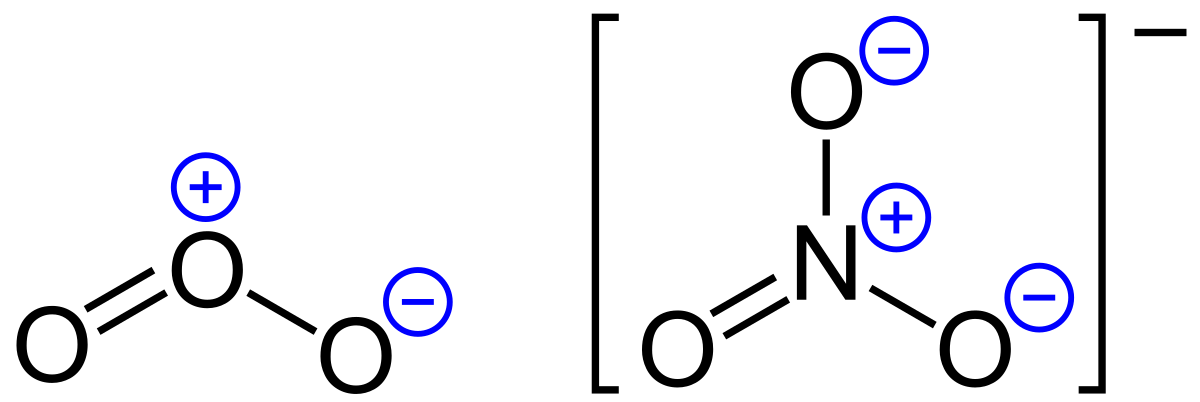
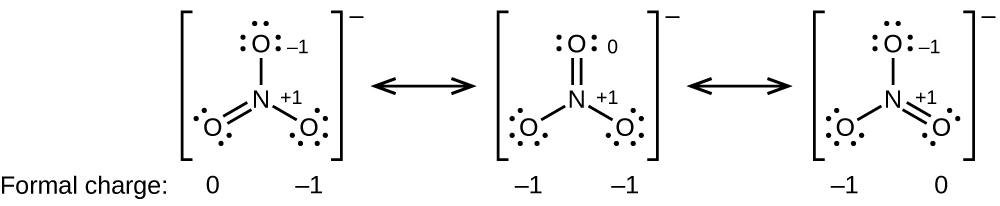
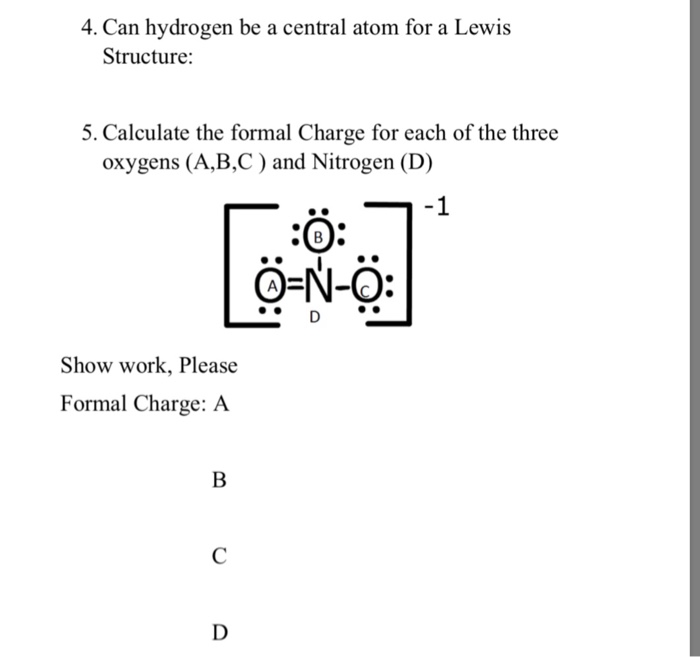
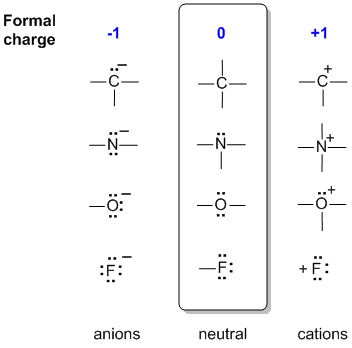
Lone pairs lone electrons sitting on the atom. They can be drawn as lines bonds or dots electrons. Using equation 231 to calculate the formal charge on hydrogen we obtain formal charge of h 1 valence e 0 lone pair e 12 x 2 bond pair e 0 the sum of the formal charges of each atom must be equal to the overall charge of the molecule or ion. How to calculate formal charge once we add all the formal charges for the atoms in the lewis structure we should get a value equal to the actual charge of the molecule or ion.
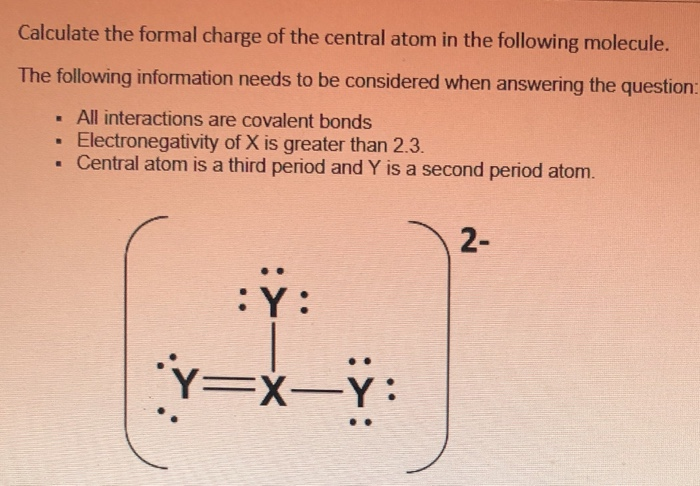
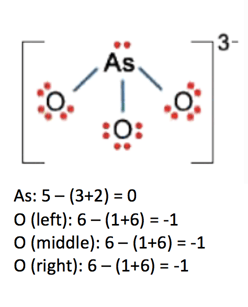
The nonbonding electrons on the other hand are the unshared electrons and these are shown as dots. Of non bonding electrons 12 x total number of shared electrons. The formal charge on an atom can be calculated using the following mathematical equation. Formal charge on p 5 12 8 0.