How To Read Periodic Table Valence Electrons
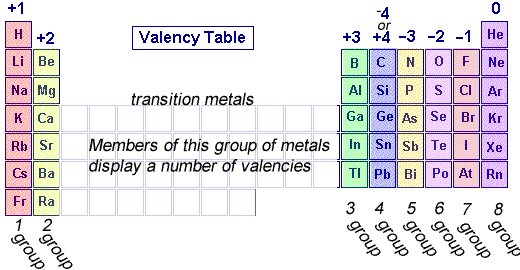
Valence electrons are the number of electrons an element has available to bond with other elements to make molecules.
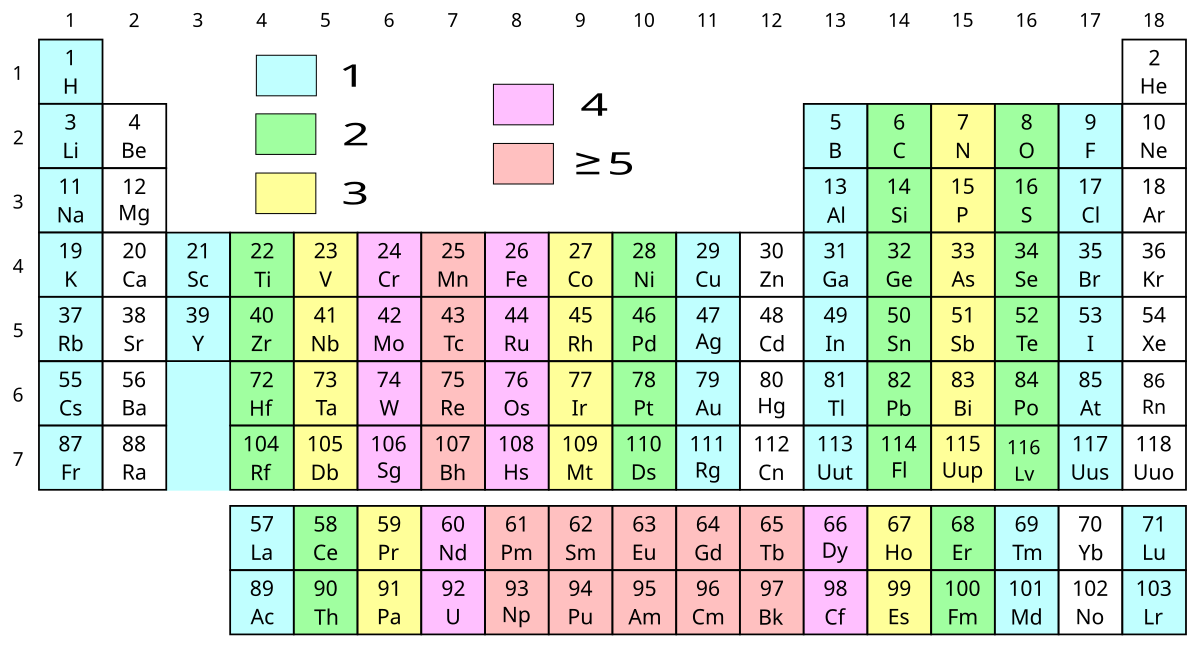
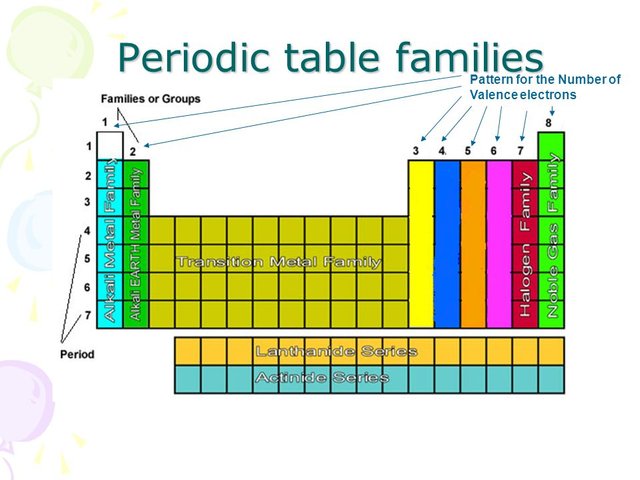
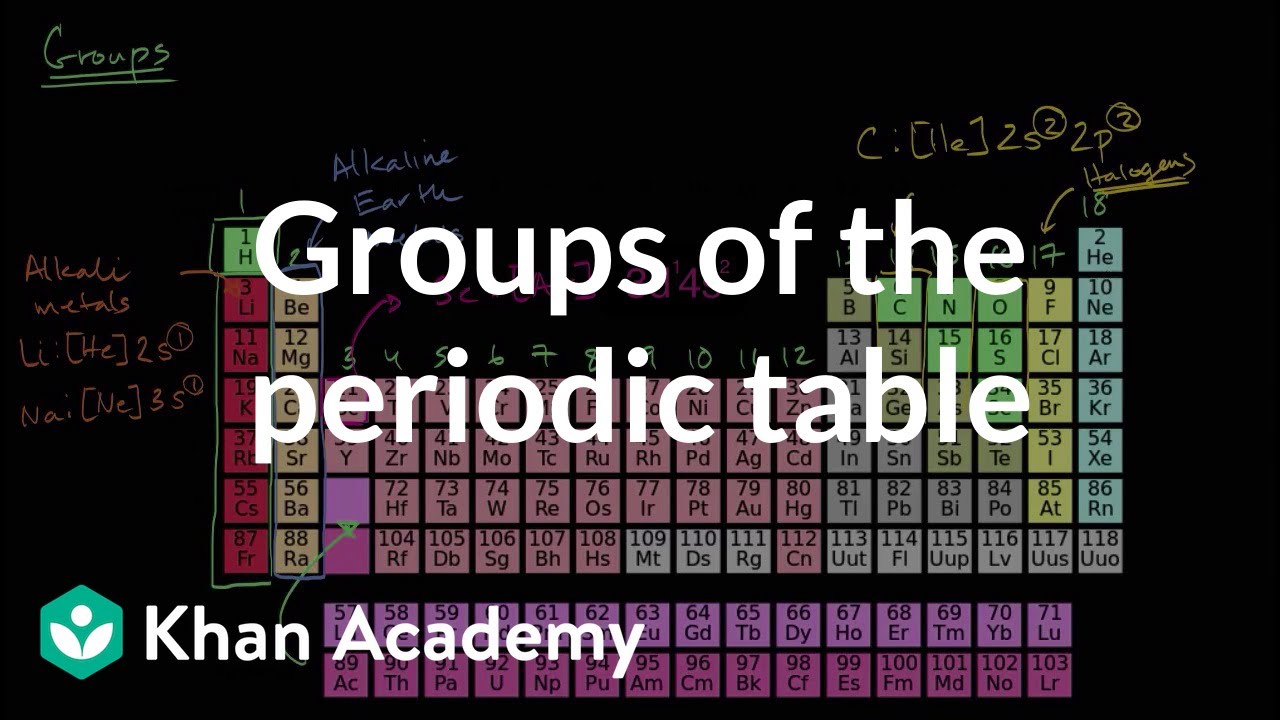
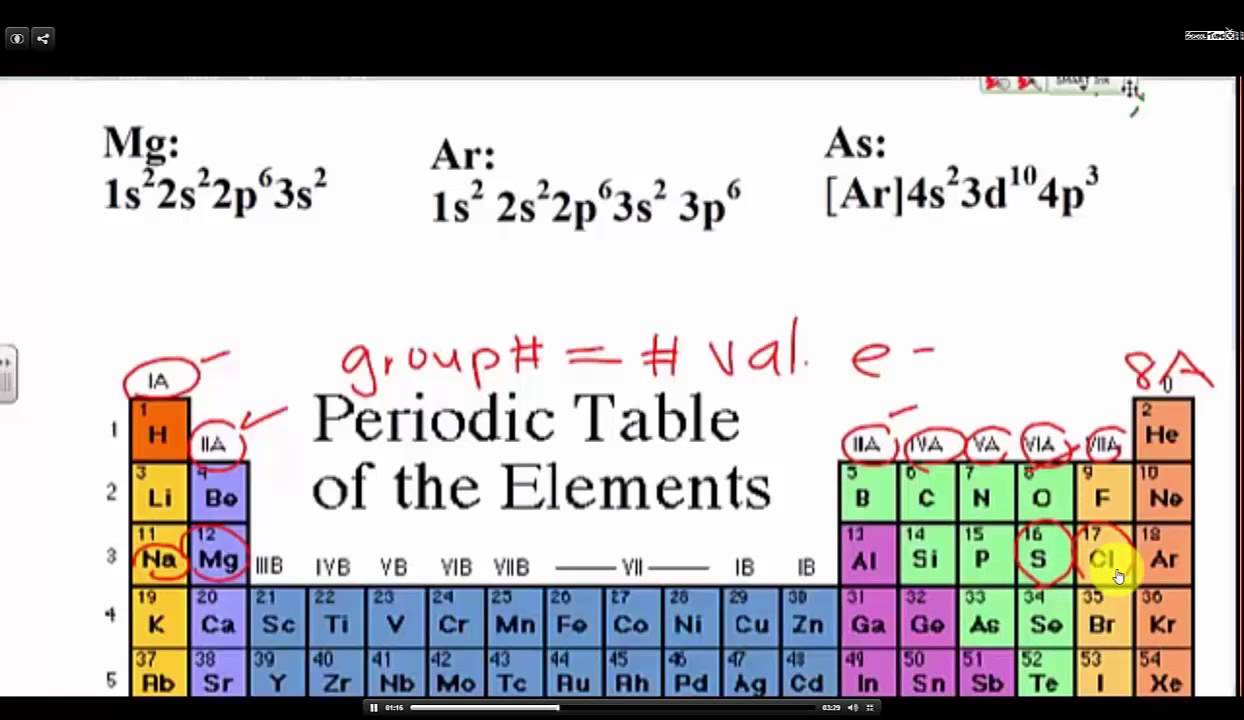
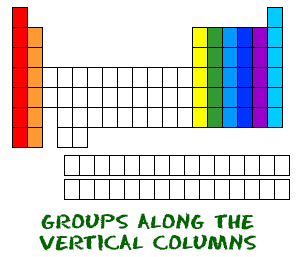
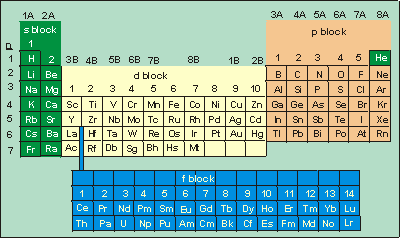
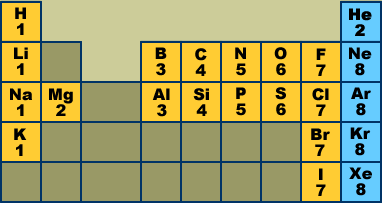
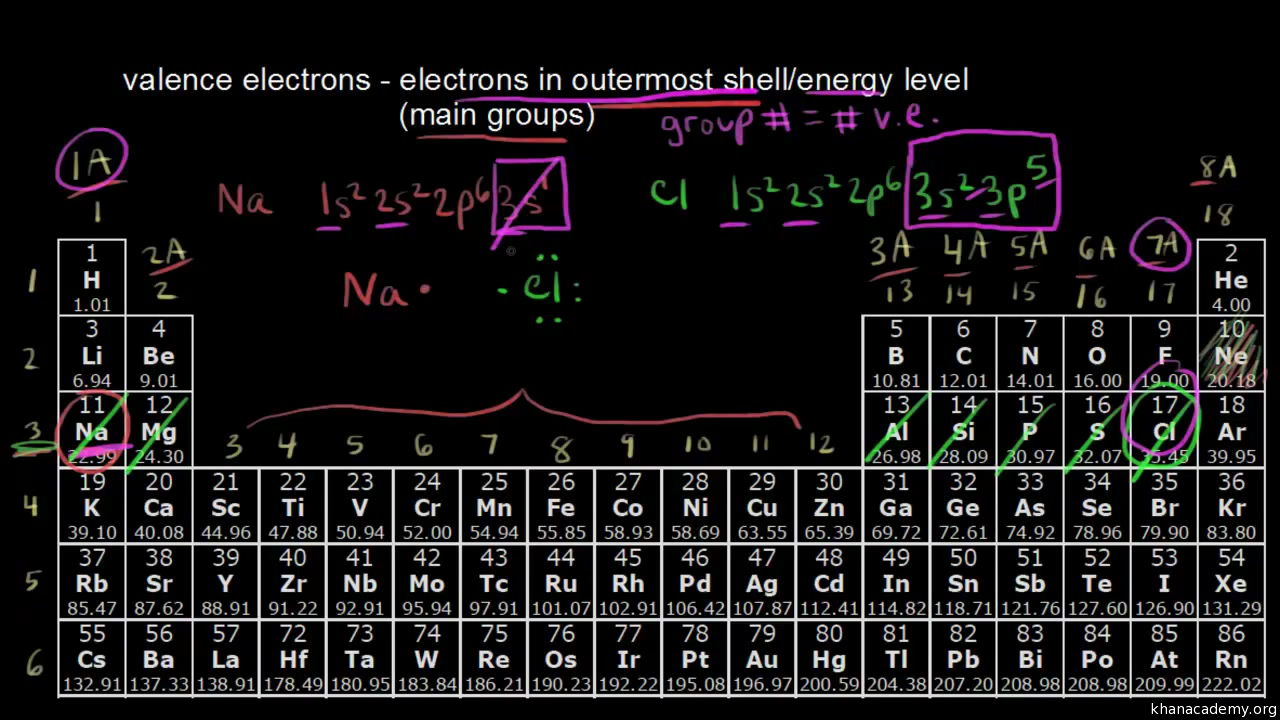
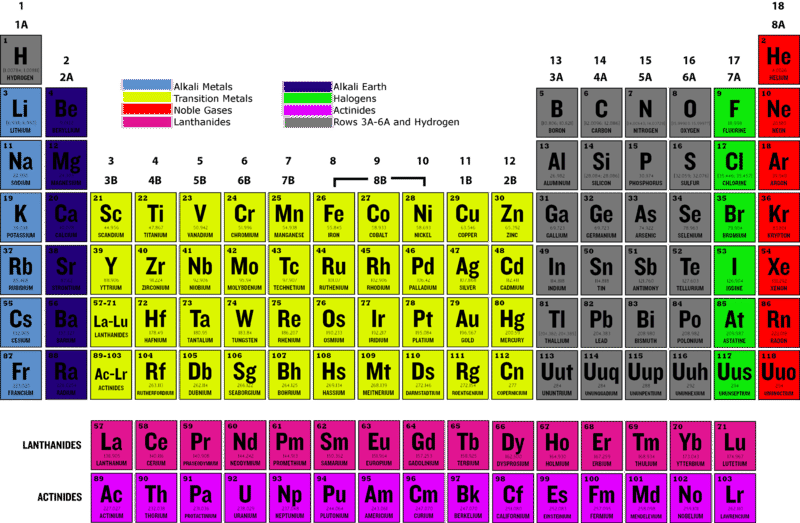
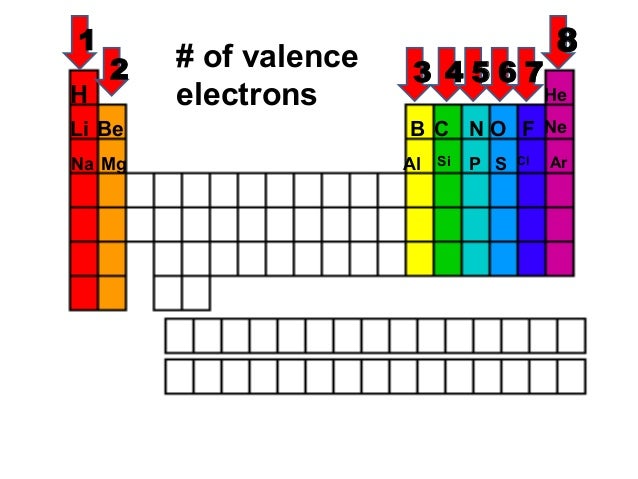
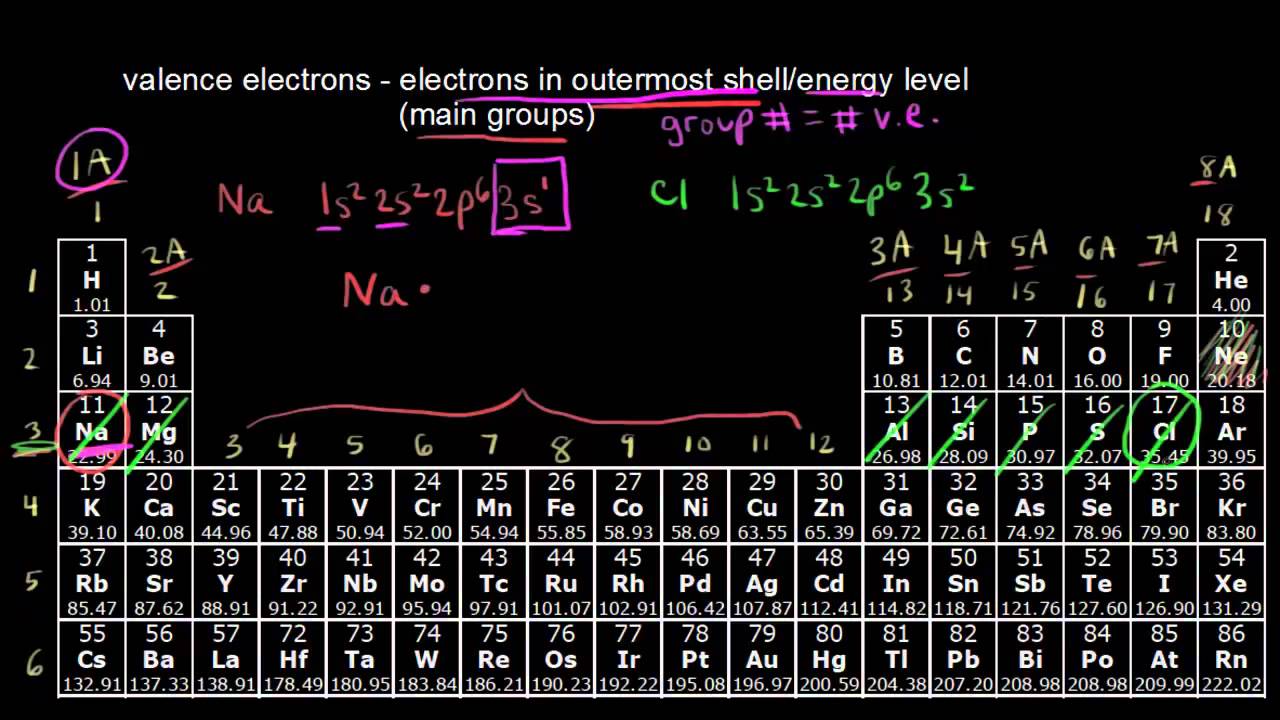
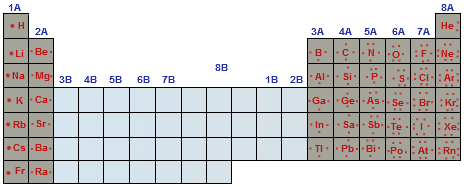
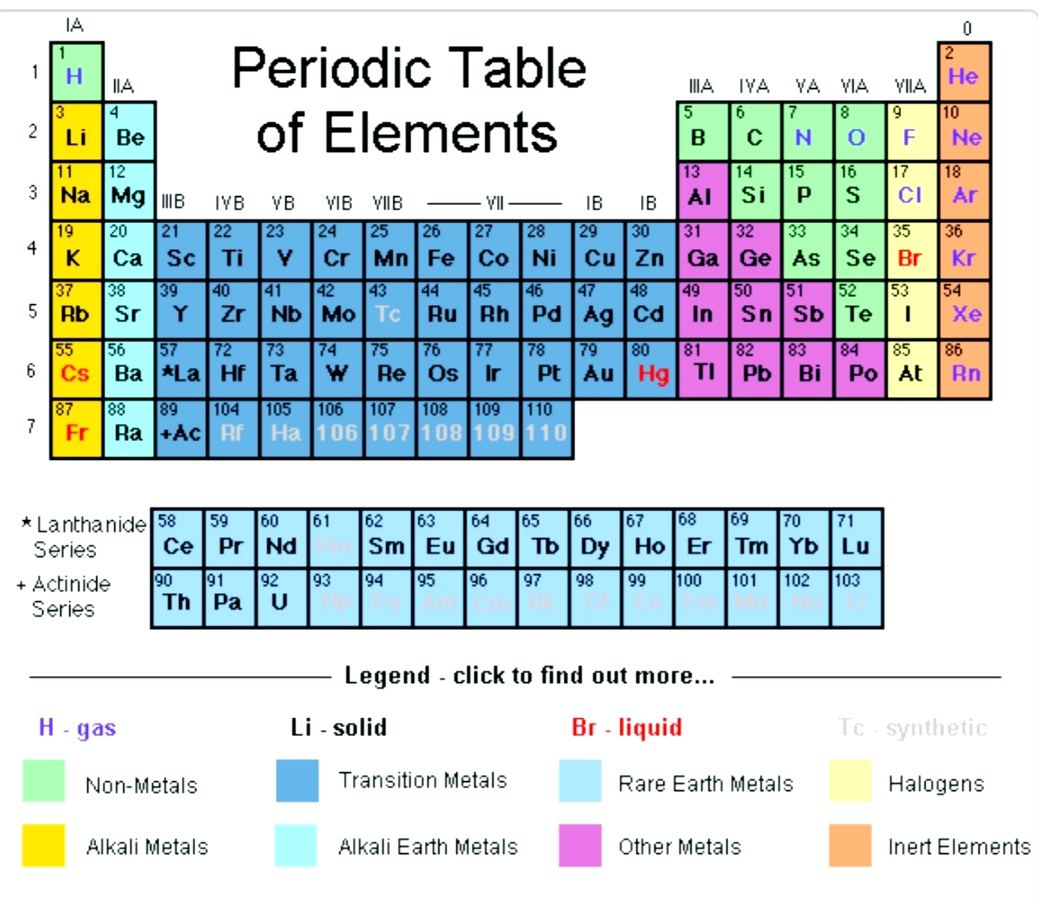
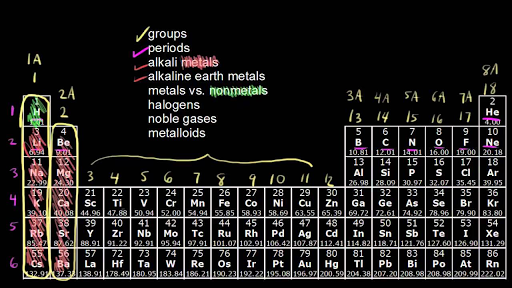
How to read periodic table valence electrons. And so for this video were only talking about the valence electrons for elements in the main groups. Generally on a periodic table all of the elements in a single vertical column will have the same number of valence electrons. The greeklatin numeral prefixes mono uni di bi tri ter and so on are used to describe ions in the charge states 1 2 3 and so on respectively. Groupscolumns on the periodic table of the elements all possess the same number of electrons in their valence shell.
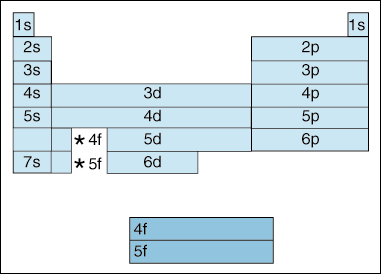
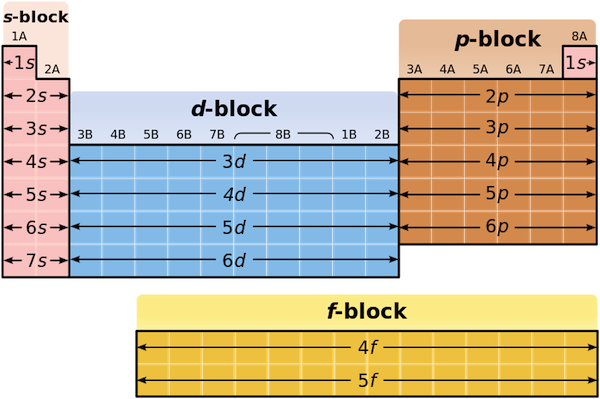
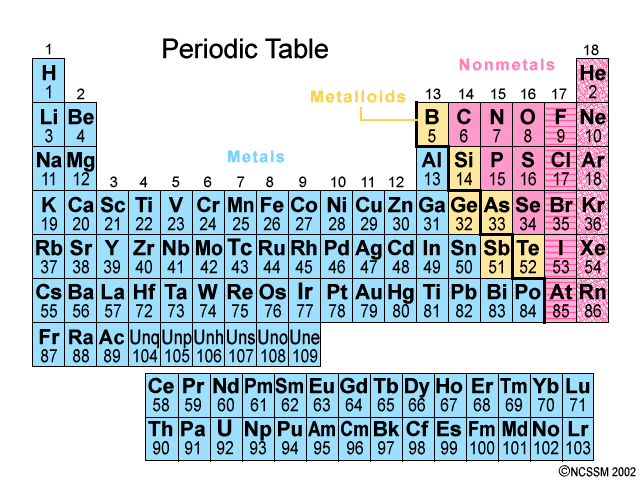
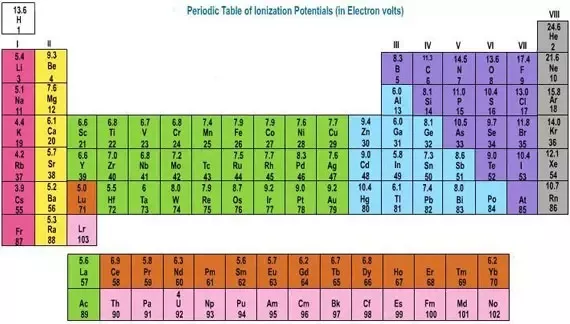
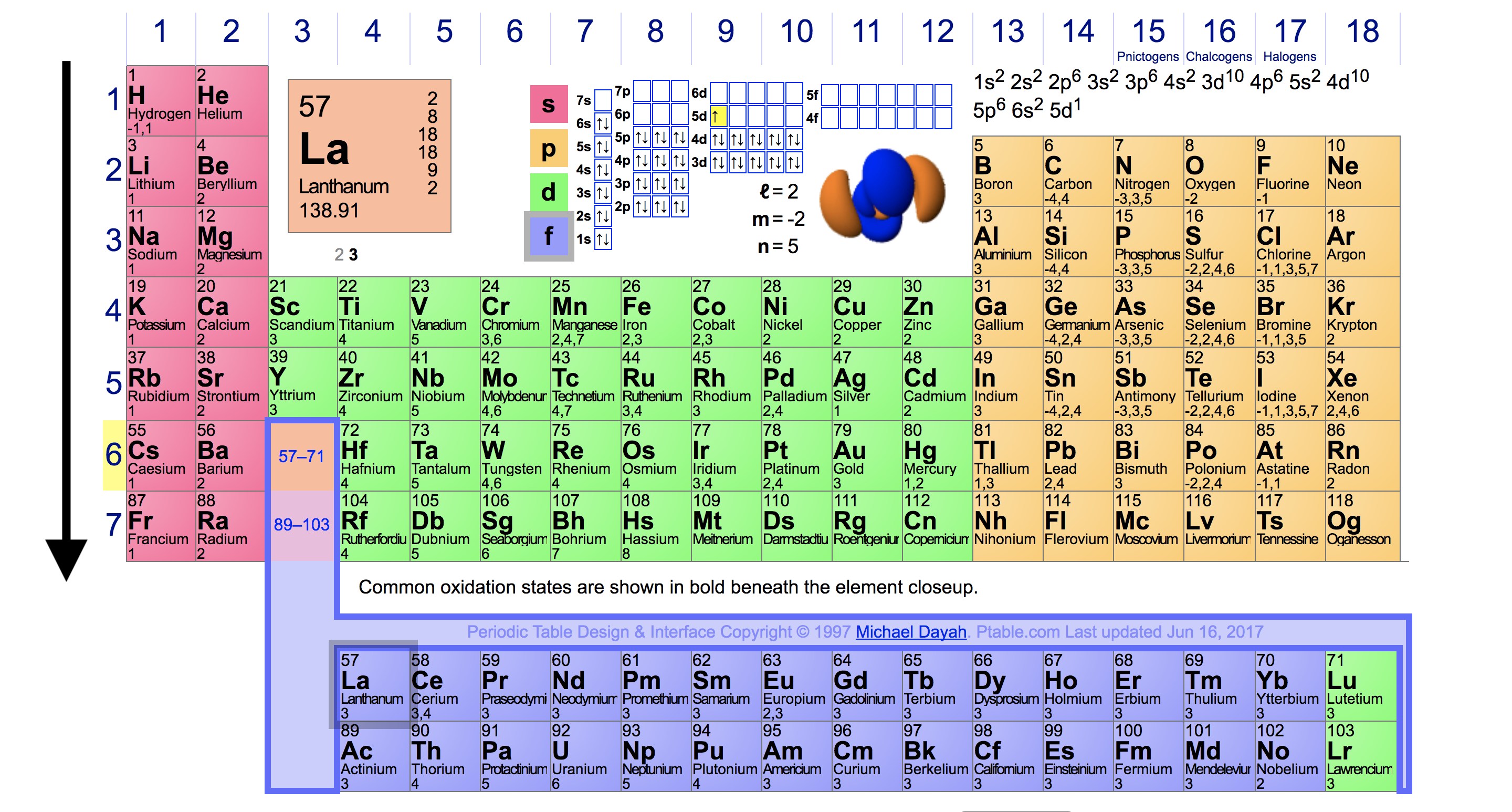
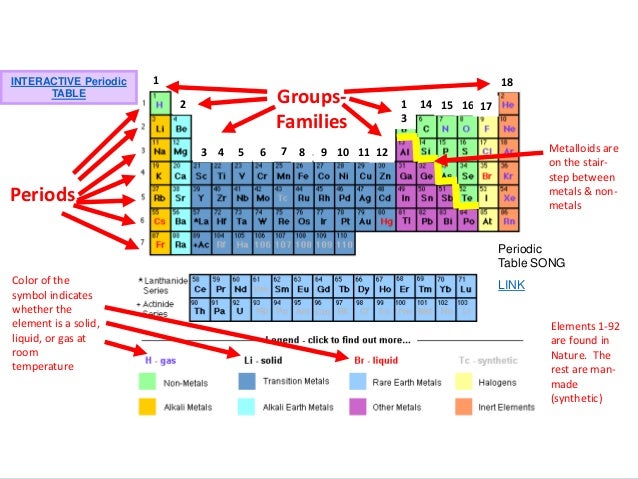
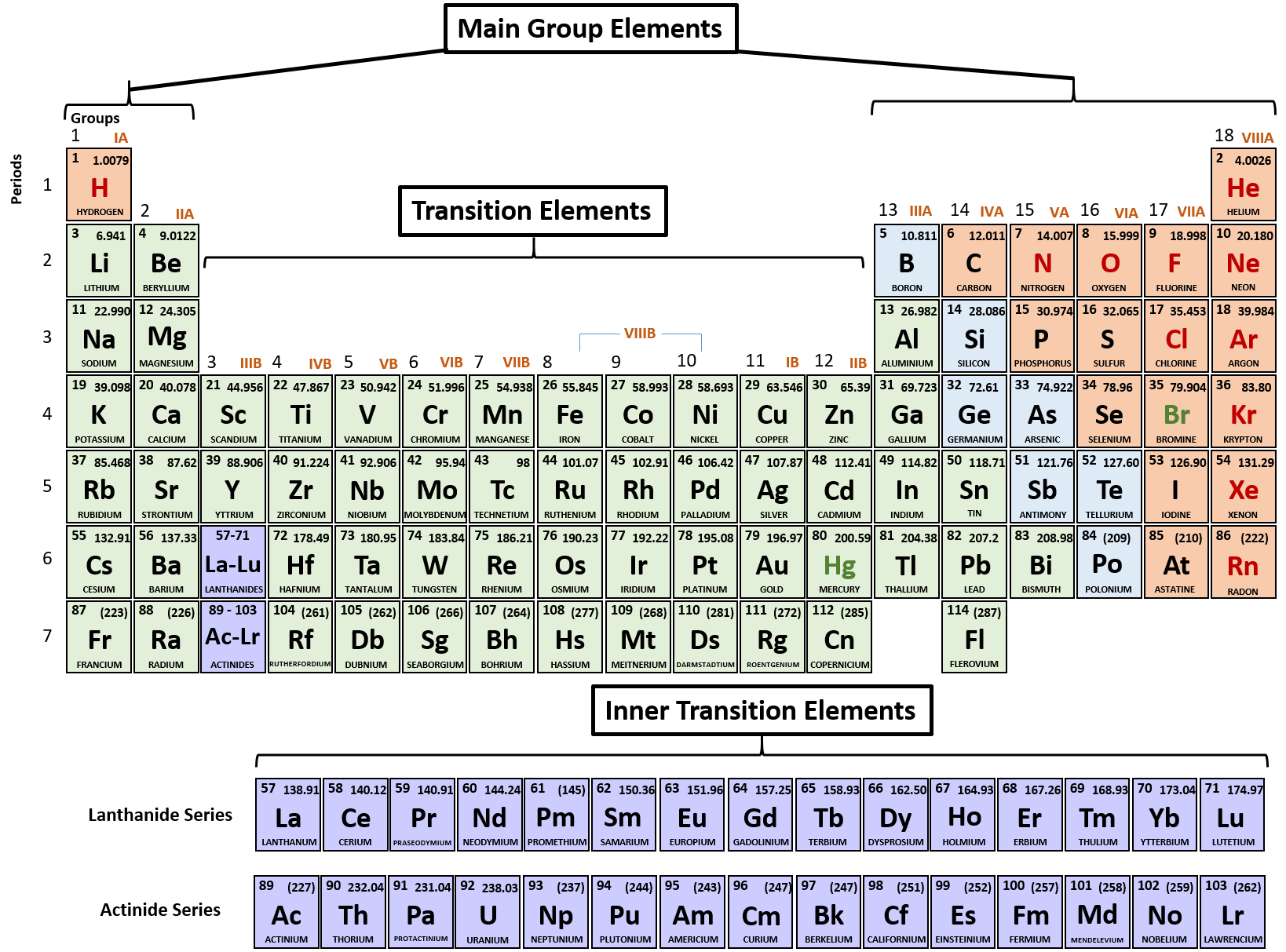
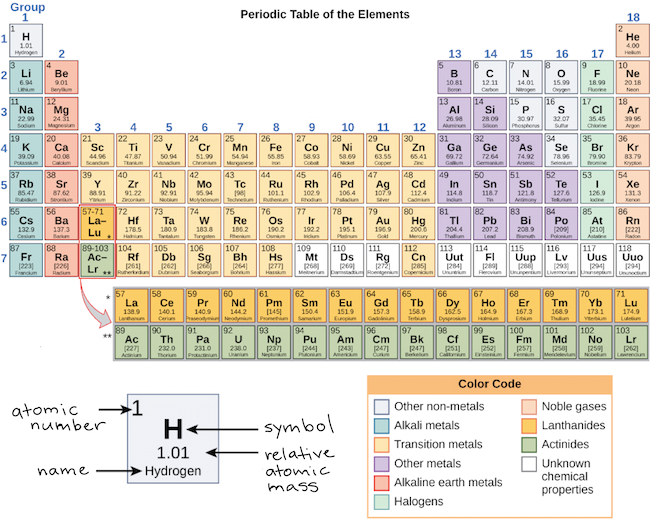
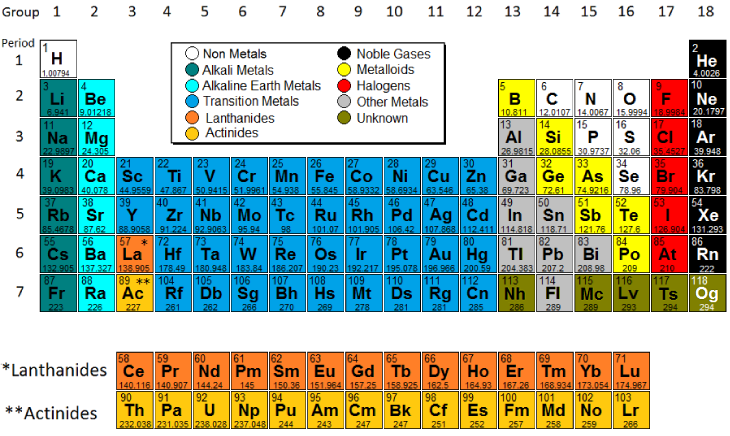
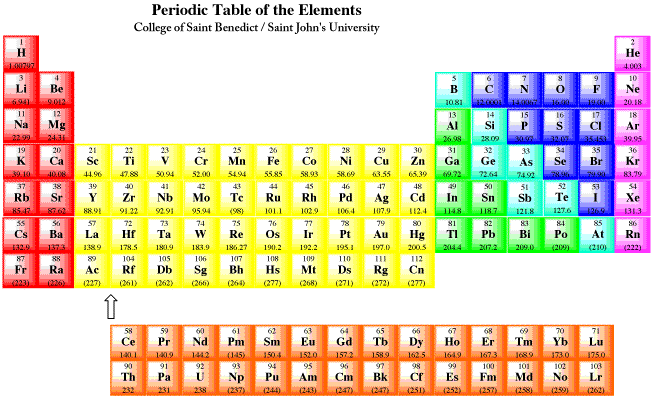
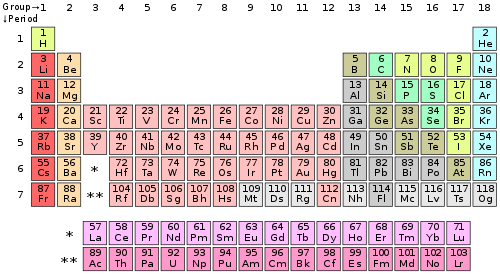
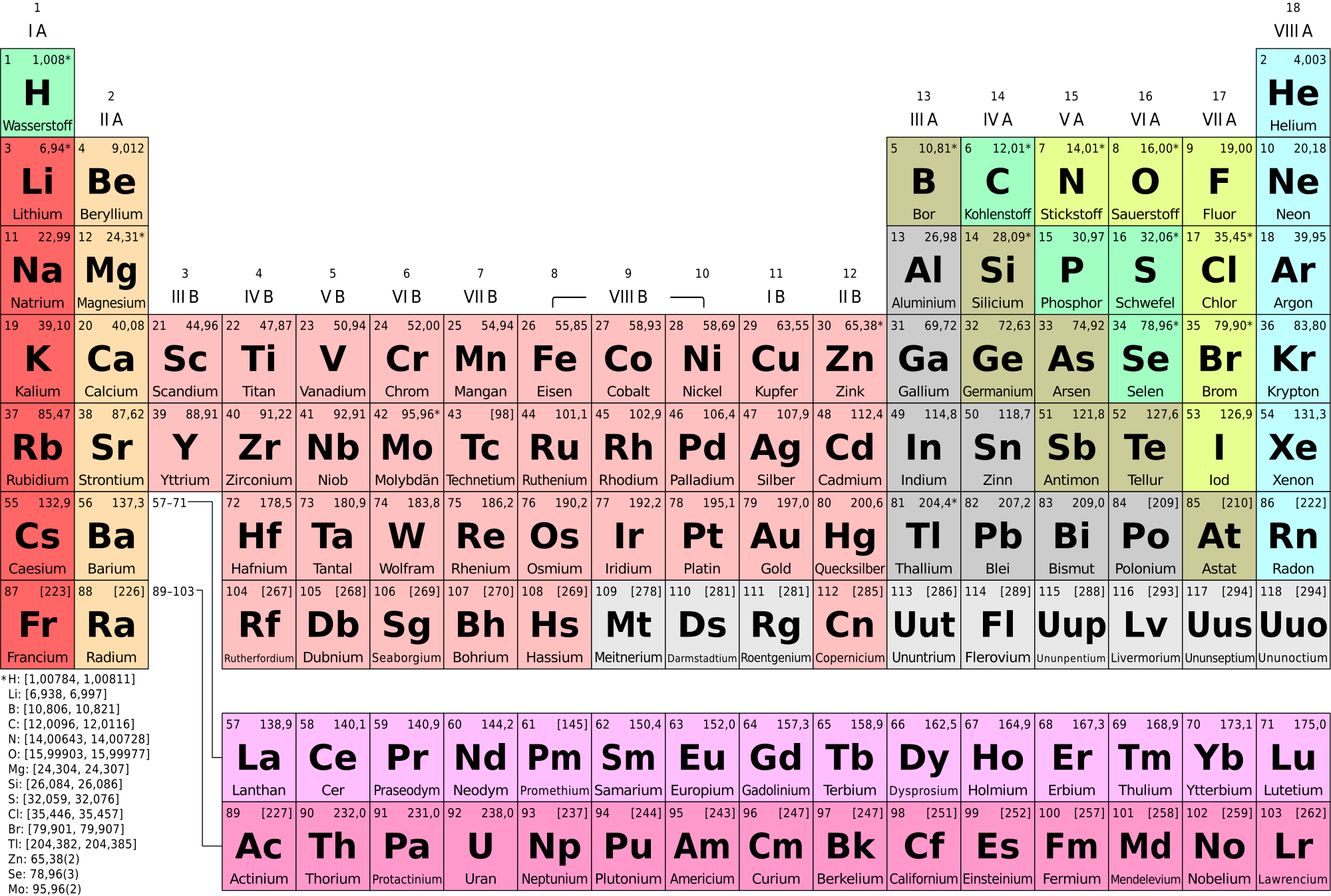
A roman numeral or in modern tables an arabic numeral designates each column. Across each row or period of the periodic table the number of valence electrons in groups 12 and 1318 increases by one from one element to the next. Now that weve classified our elements into groups on the periodic table lets see how to determine the number of valence electrons. The number of valence electrons in an atom is reflected by its position in the periodic table of the elements see the periodic table in the figure below.
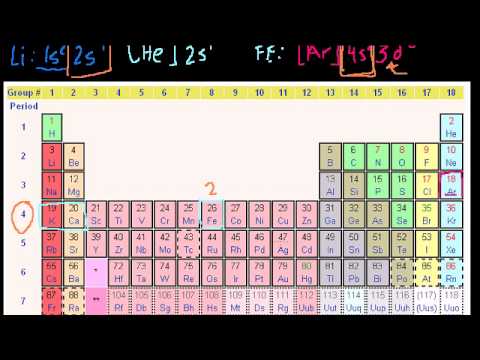
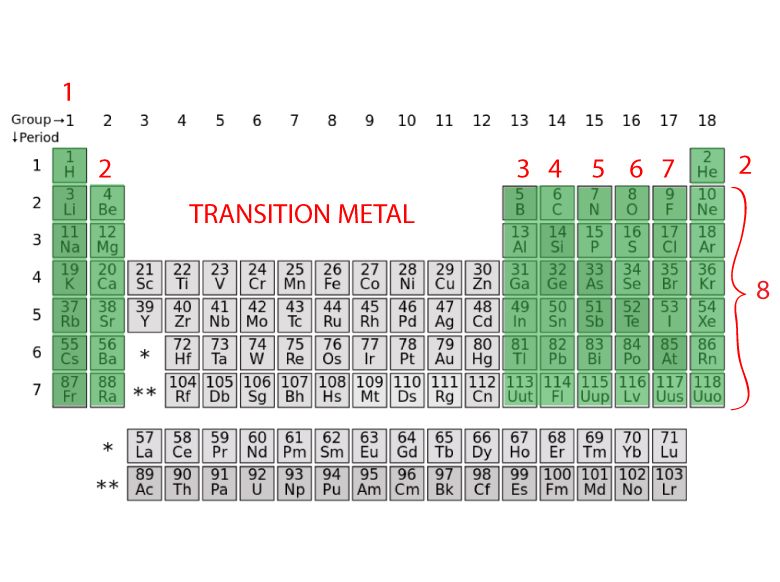
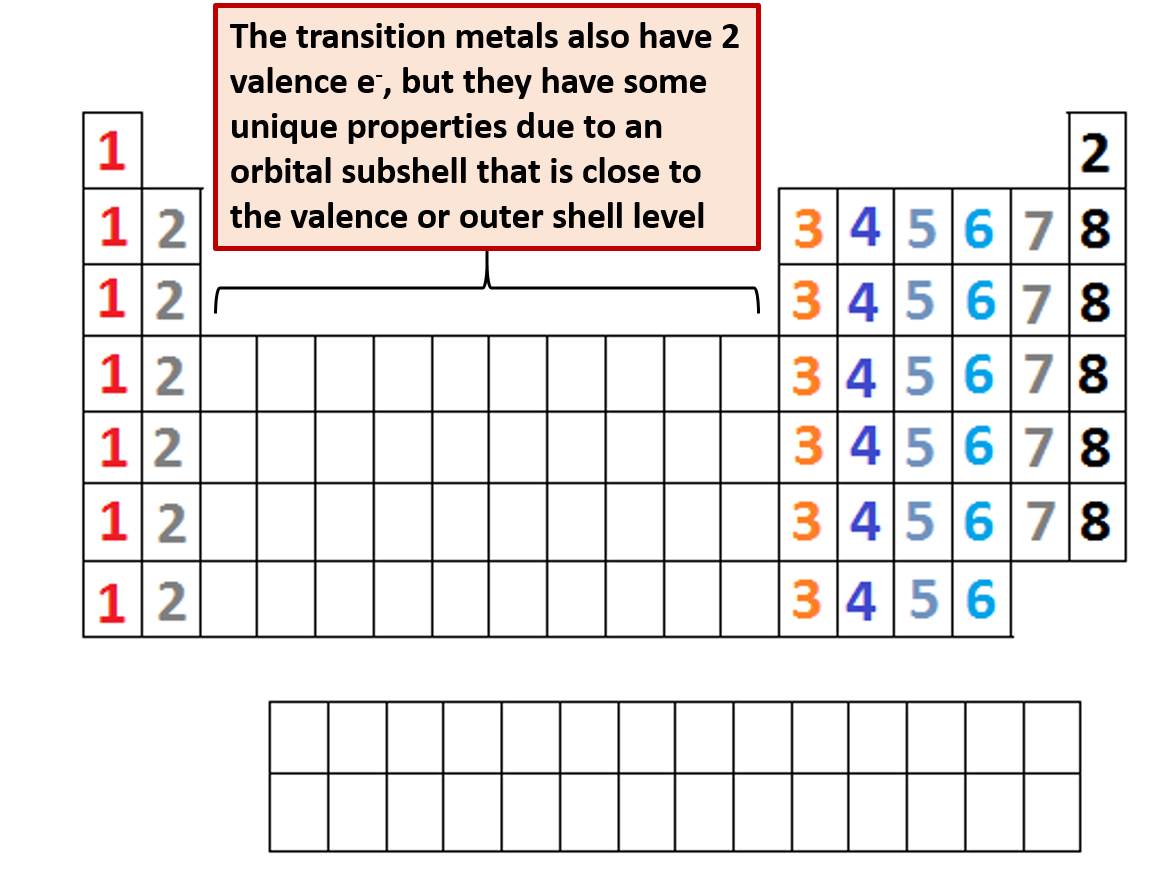
This can be confirmed by checking the number of valence electrons in the first group of the periodic table. This is the number of electrons an element has in its outer shell which are known as valence electrons. With the exception of groups 312 the transition metals the units digit of the group number identifies how many valence electrons are associated with a neutral atom of an element listed under that particular column. Finding valence electrons for all elements except transition metals locate the desired element on the periodic table.
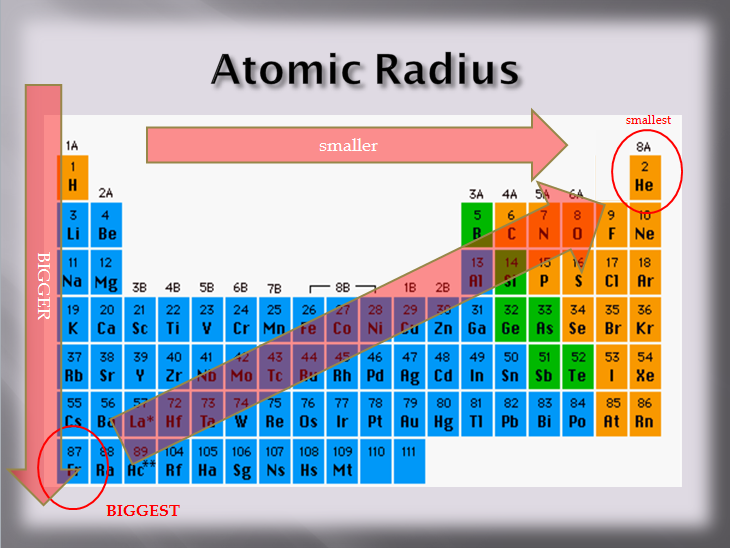
In scientific terms these columns are called the element groups. For example locate the element oxygen on the table. The elements are ordered by their atomic numbers which increase as you move across and down the periodic table. The atomic number is how many protons the elements atom possesses.
Group one of the periodic table contains elements like sodium potassium hydrogen and cesium. If your periodic table doesnt already have each column numbered give each a number starting with 1 for the far left end and 18 for the far right end. The periodic table of the chemical elements. When we talk about the main groups youre using the one through eight system for classifying groups.












/PeriodicTableWallpaper-56a12d103df78cf7726827e81-0c02b1ee4c8b42478b651c7c9aaac7f9.jpg)










/186810031-56a130cd5f9b58b7d0bce8ee.jpg)





/chart-of-periodic-table-trends-608792-v1-6ee35b80170349e8ab67865a2fdfaceb.png)







/PeriodicTableWallpaper-56a12d103df78cf7726827e81-0c02b1ee4c8b42478b651c7c9aaac7f9.jpg)