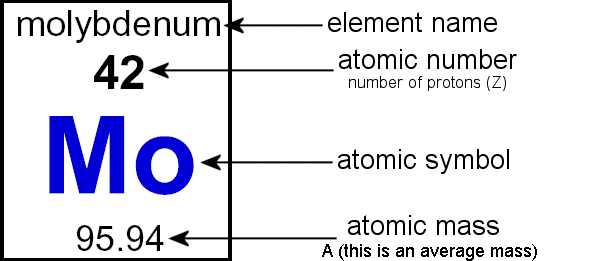
How To Find The Mass Of An Isotope
Find out how isotopes can be detected using mass spectrometry.
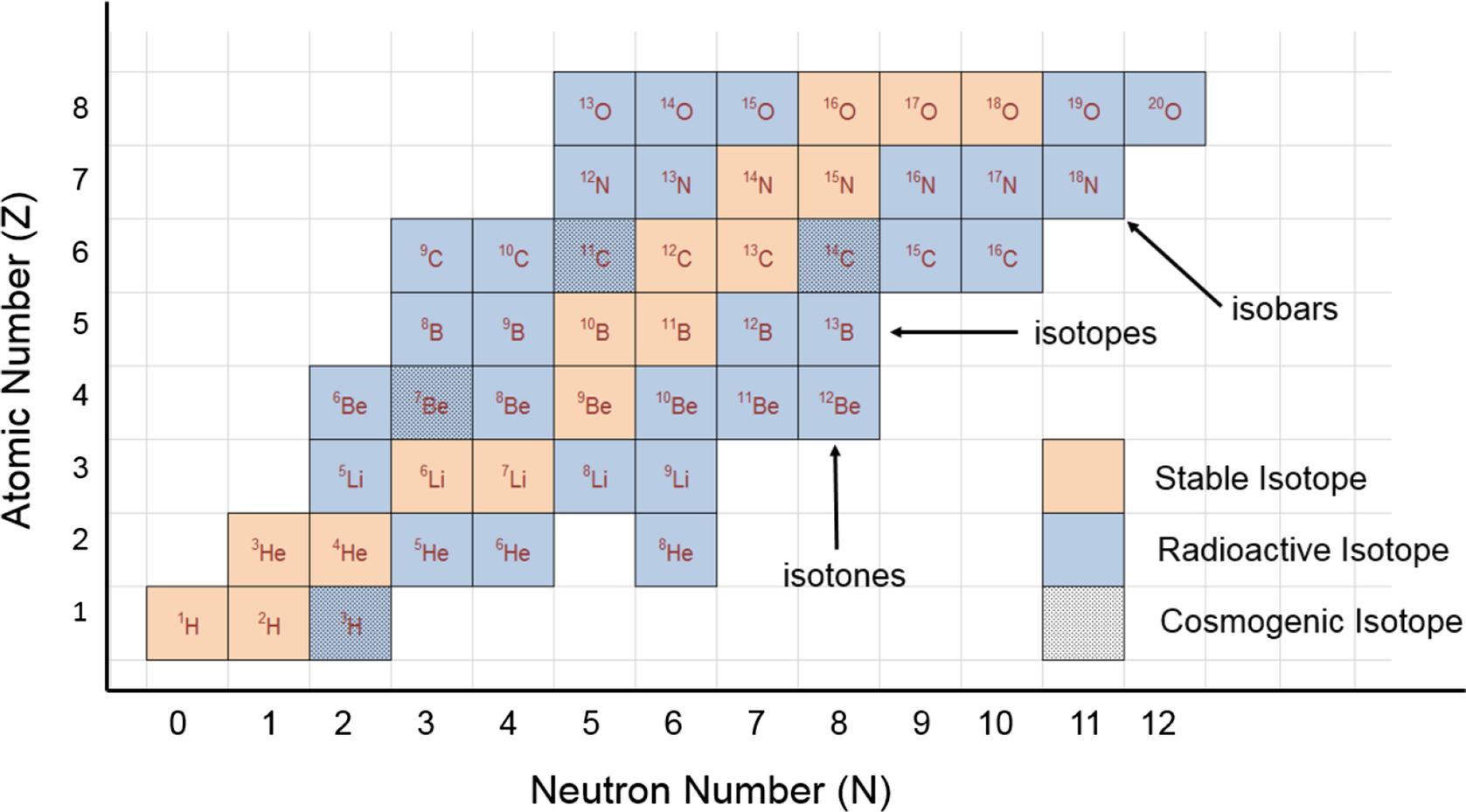
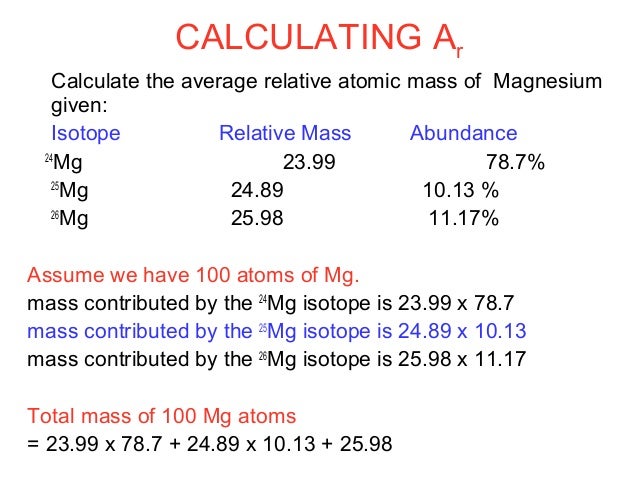
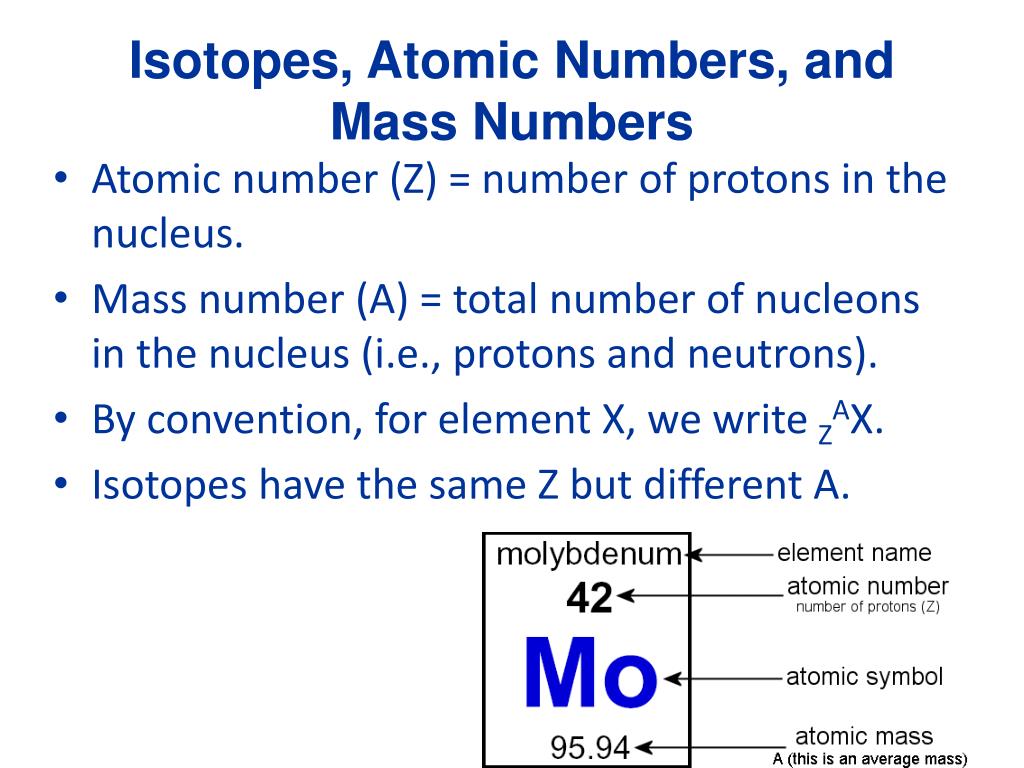
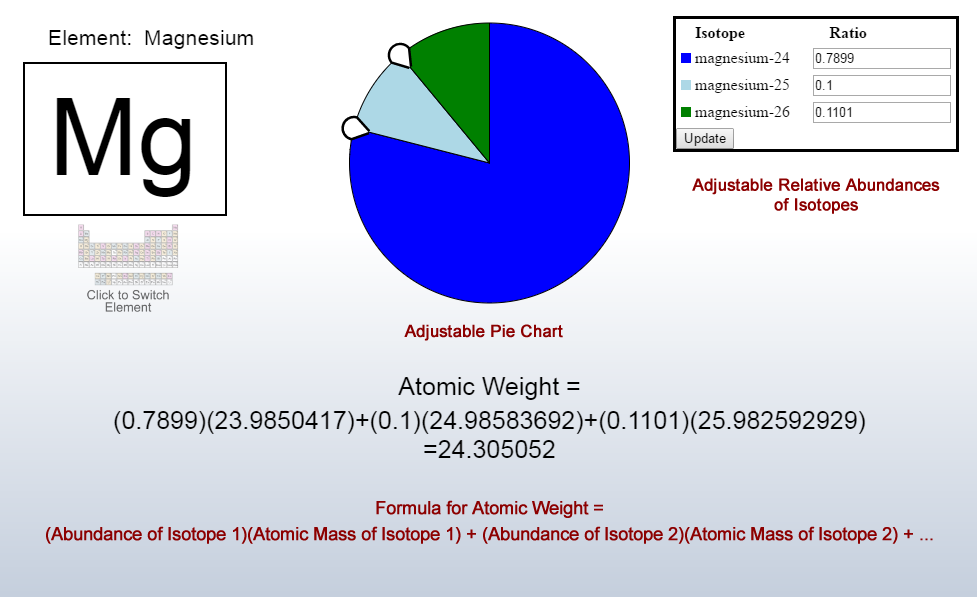
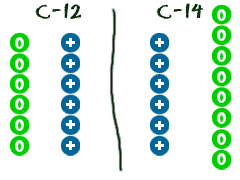
How to find the mass of an isotope. Add the number of neutrons to the number of protons to find the nominal mass or mass number. Because mass spectrometry determines the weights of fragments atoms that naturally have heavy isotopes become important. Total mass 7899 23985 amu 1000 24986 amu 1101 x. Mass spectrometry provides valuable information about the structure of a molecular compound including its isotopes.
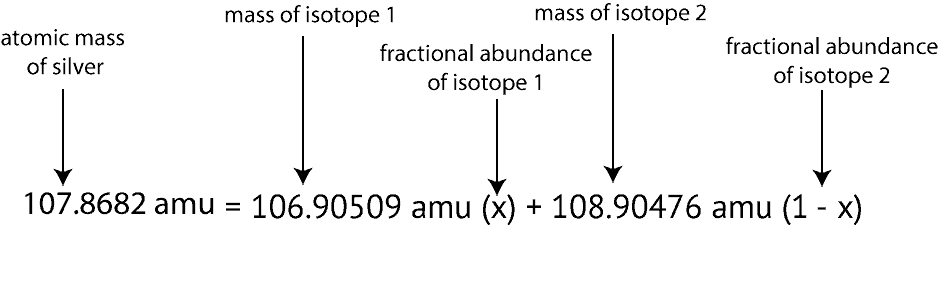
The mass number of carbon 13 for example is 13. The average mass of the element e can be expressed as. 01101 x 243051. In the equation a is the average atomic mass b is the atomic mass of one isotope c is the atomic mass of the other isotope and x is the abundance of the first isotope.
Me sumn1min times pin. For example 10811 10013 x 11009 1 x. The converted percentages should always add up to 1. On the basis of the abundance of isotopes we can calculate the isotopic mass and average atomic mass of an element.
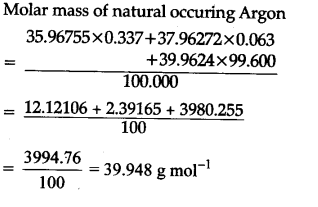
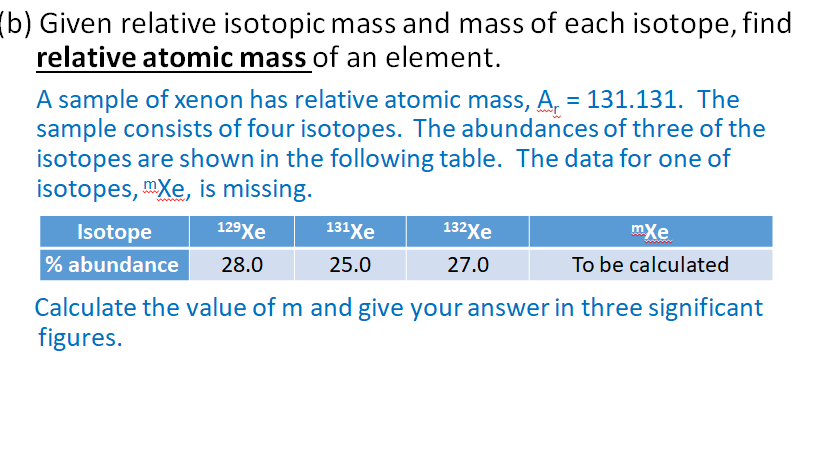
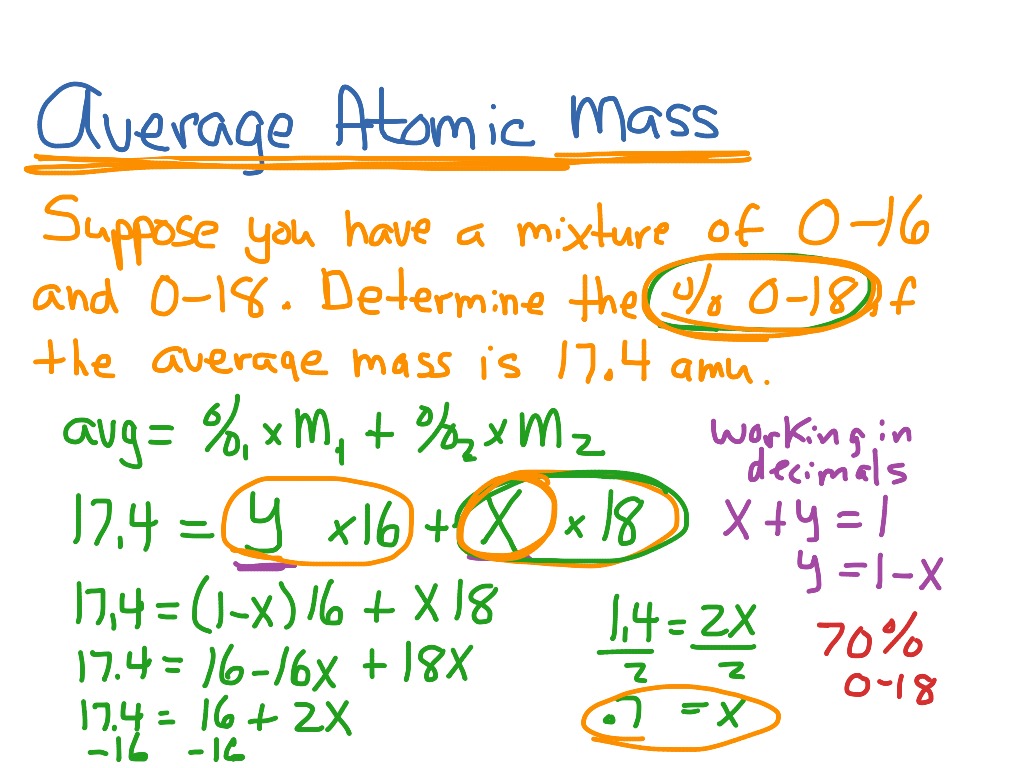
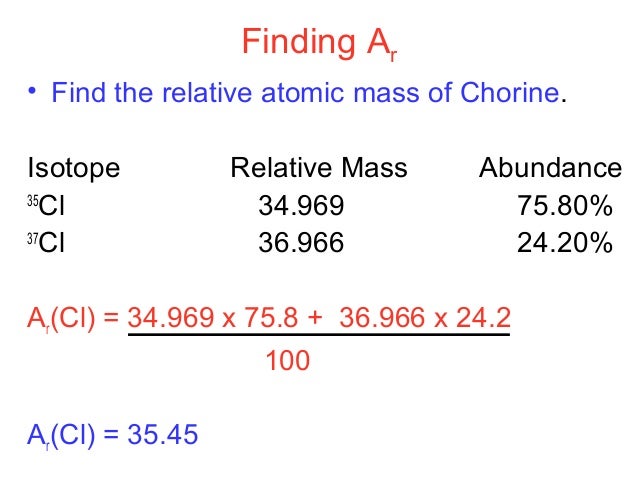
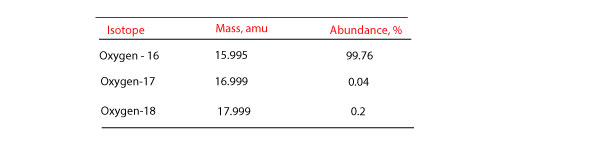
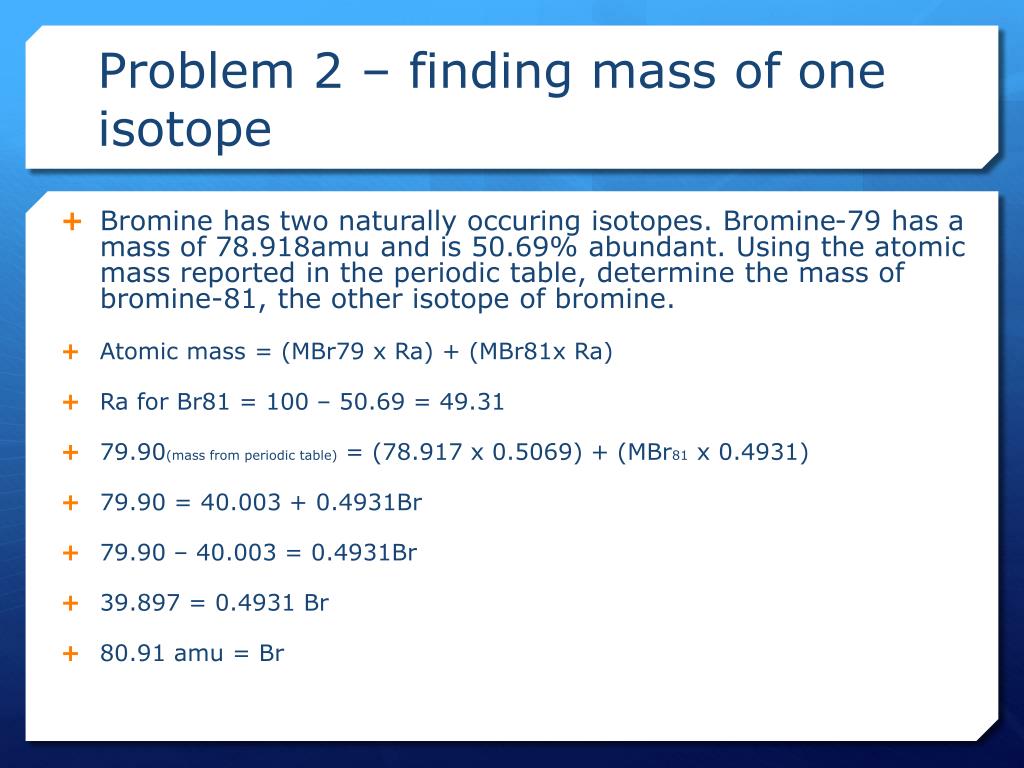
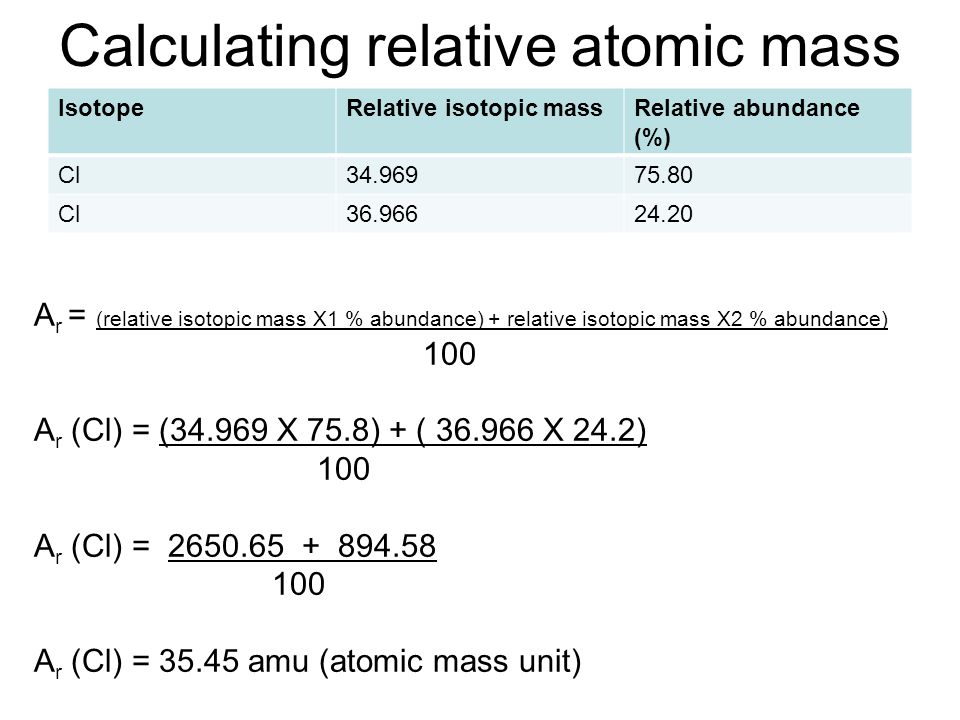
Find the atomic mass of second isotope when atomic mass and mass percent of other isotope is given submitted by djgraci on tue 09272011 0421 antimony sb which has an atomic weight of 12175 amu has two naturally occuring isotopes. For most calculations the nominal mass should be sufficient. 243051 gmol 07899 23985 amu 01000 24986 amu 01101 x. Isotopes with more neutrons have more mass.
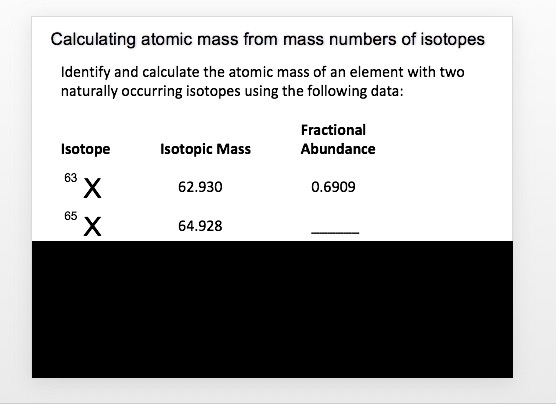
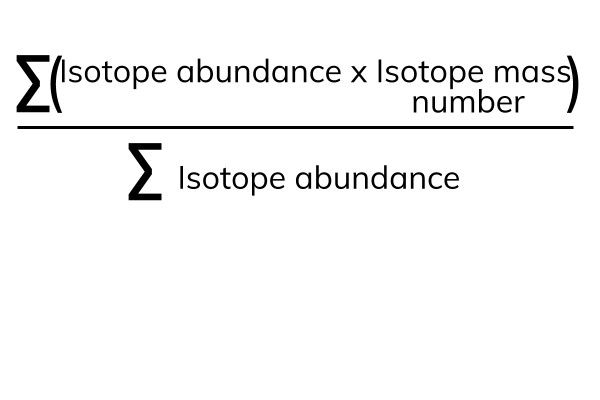
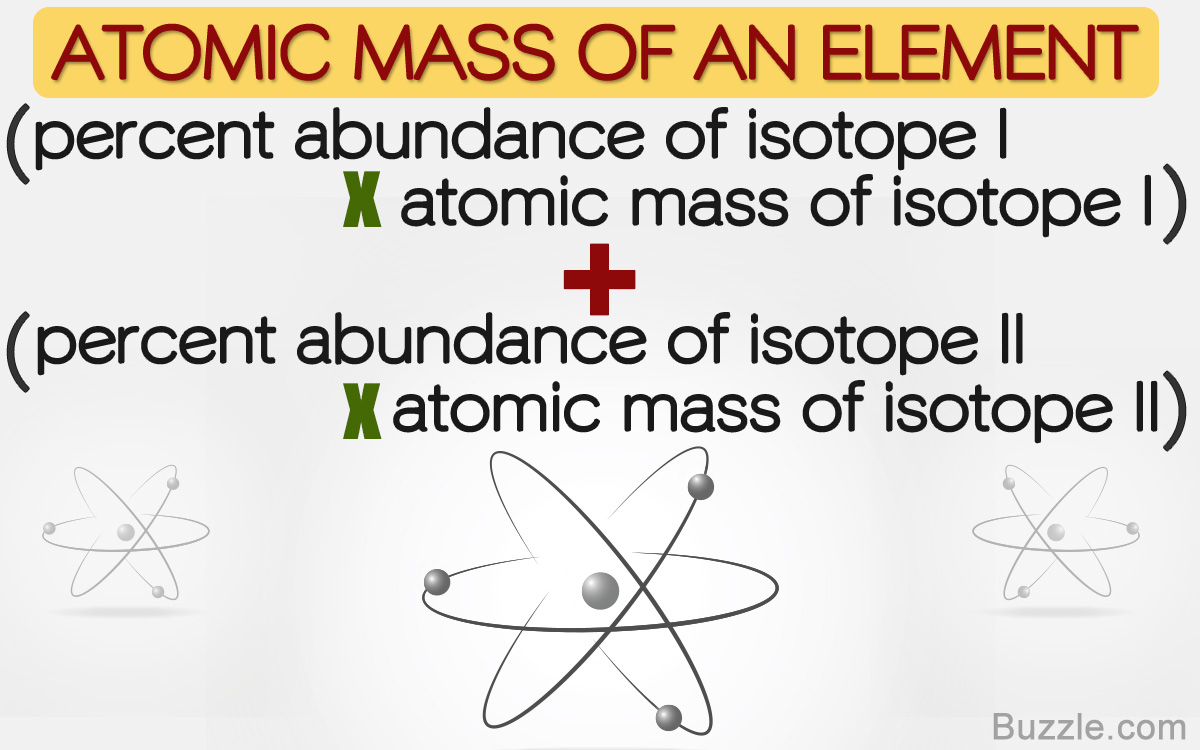
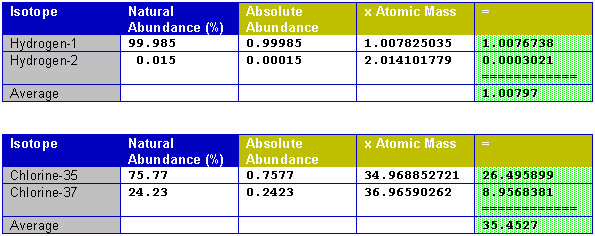
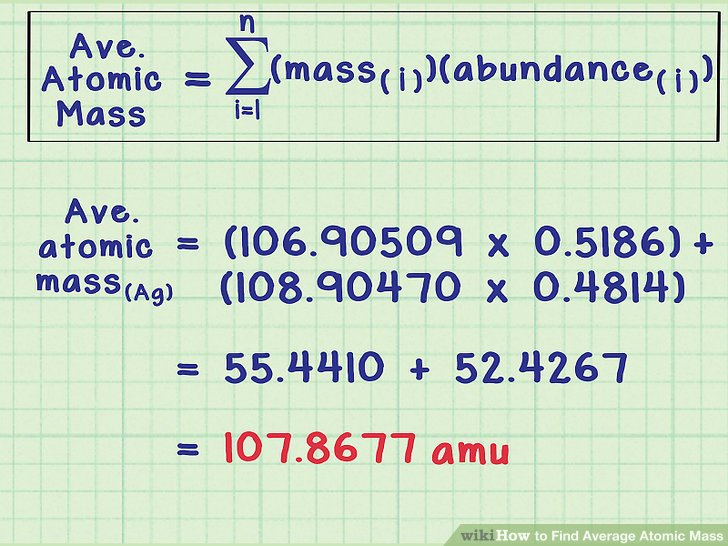
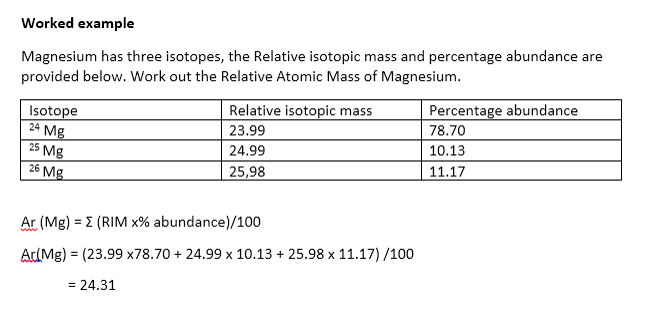
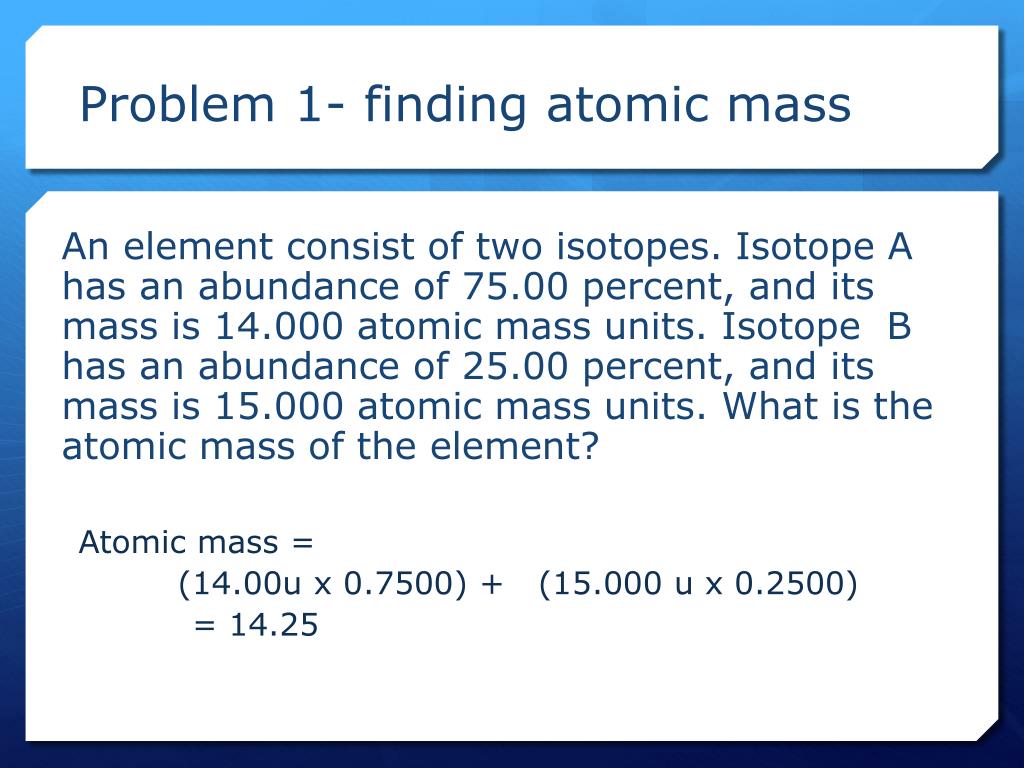
Average mass of an element atomic mass of isotope i x percent abundance of isotope i100 atomic mass of isotope ii x percent abundance of isotope ii100 in order to find the abundance of an element its necessary to compute the average of atomic masses of its isotopes in the first place. Multiply the atomic mass of each isotope by its proportion in the sample. Keep in mind that owing to the binding energy for nucleons the actual mass of carbon 13 will be very slightly different from the nominal mass. Multiply the atomic mass of each isotope by its percent abundance written as a decimal.
243051 18946 24986 01101 x. To convert a percentage to a decimal simply divide it by 100. Enter the values into the following formula. Look up the mass of each isotope.
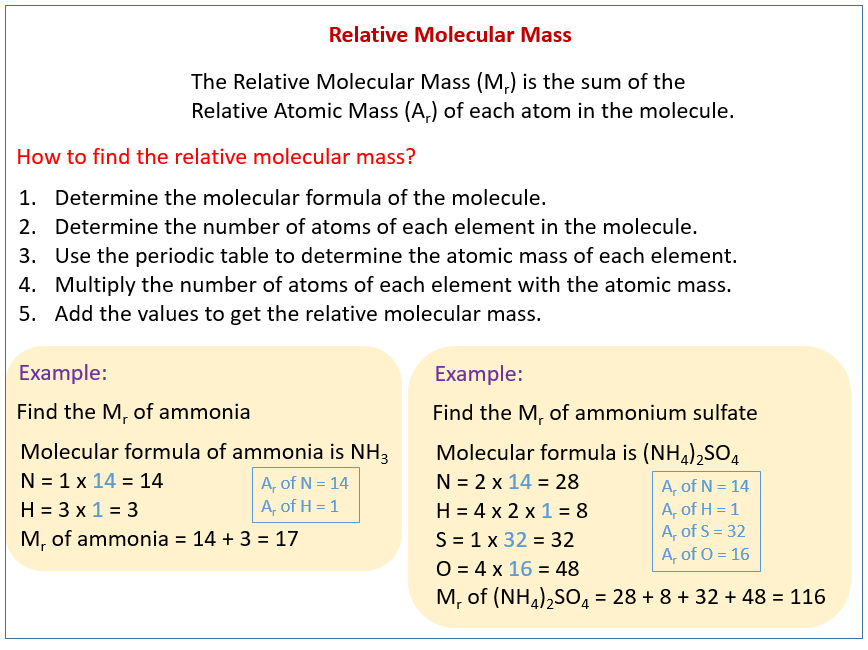
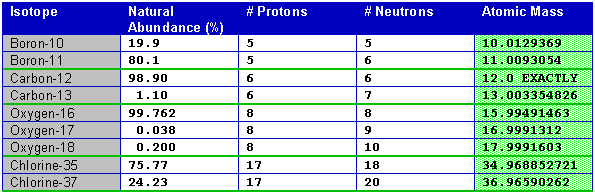
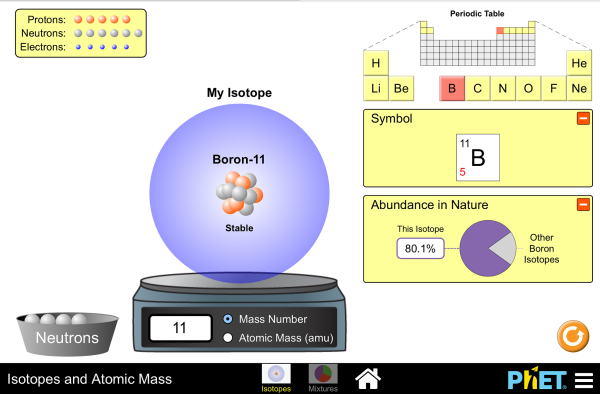
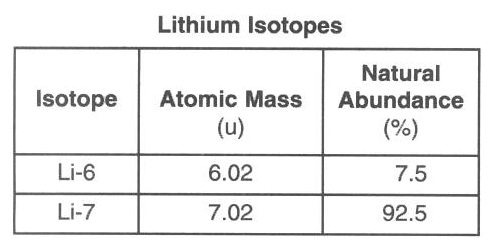
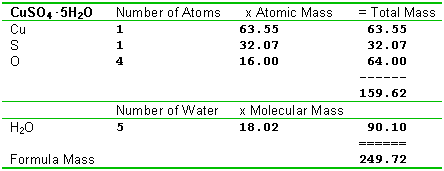
For example the atomic mass of lithium is 6941 da. For example the mass and abundance of isotopes of boron are given below. The first is the atomic mass or the mass of one atom of each isotope.



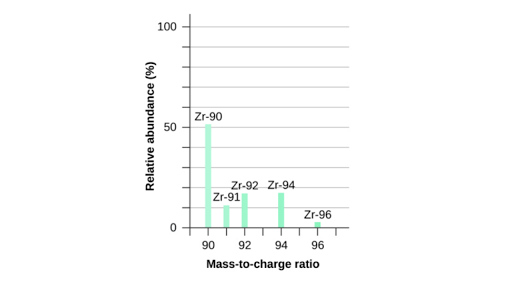


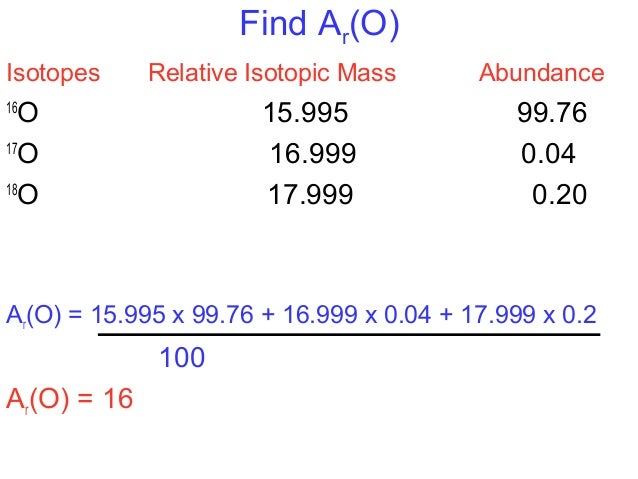

/atomic-weight-and-atomic-mass-difference-4046144_FINAL_STILL-5940e35000b145ba83fb8e3e40792ba9.png)







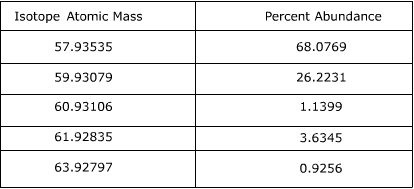



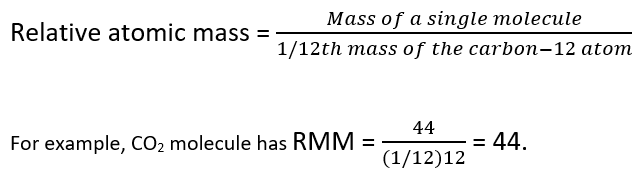

















:max_bytes(150000):strip_icc()/atomic-mass-and-mass-number-606105_v1-80df956ab98440bc9969531d1bb6c874.png)
/atomic-mass--58dc0d885f9b58468332c41b.jpg)