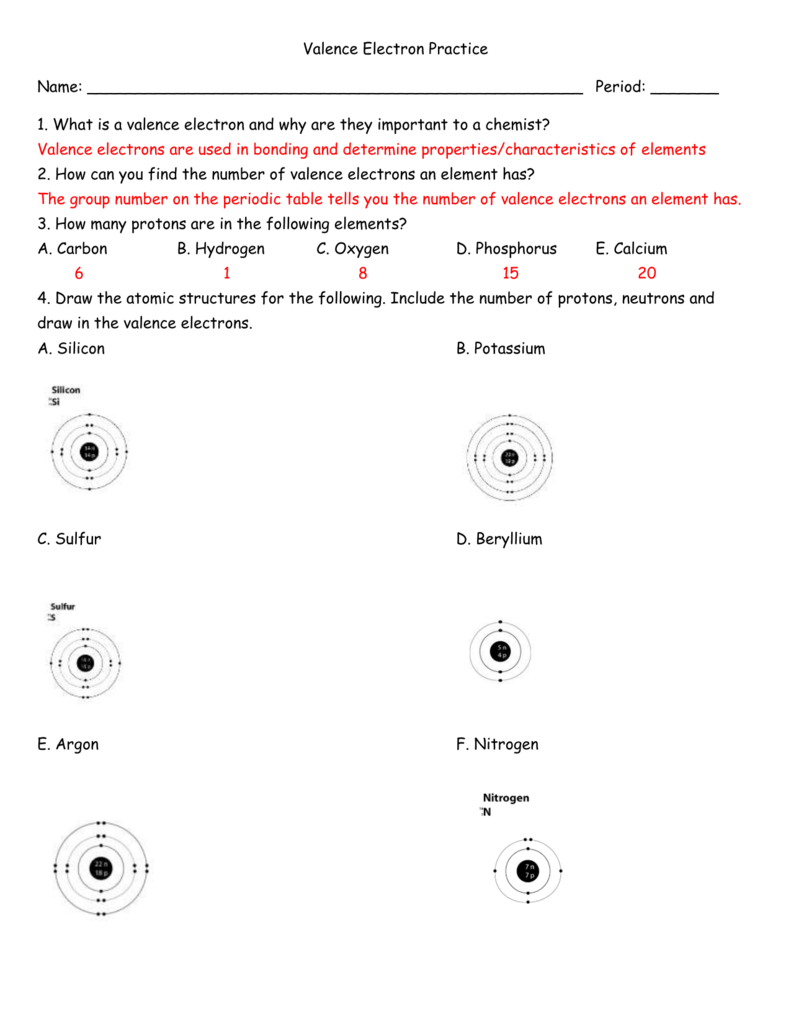
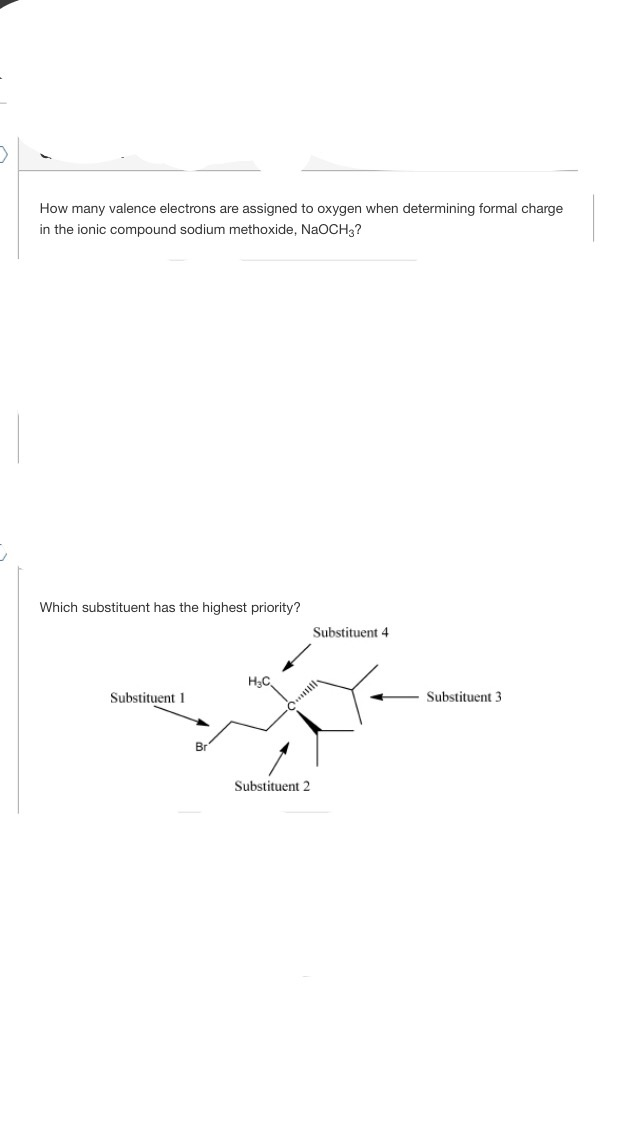
How To Find Number Of Valence Electrons In A Compound
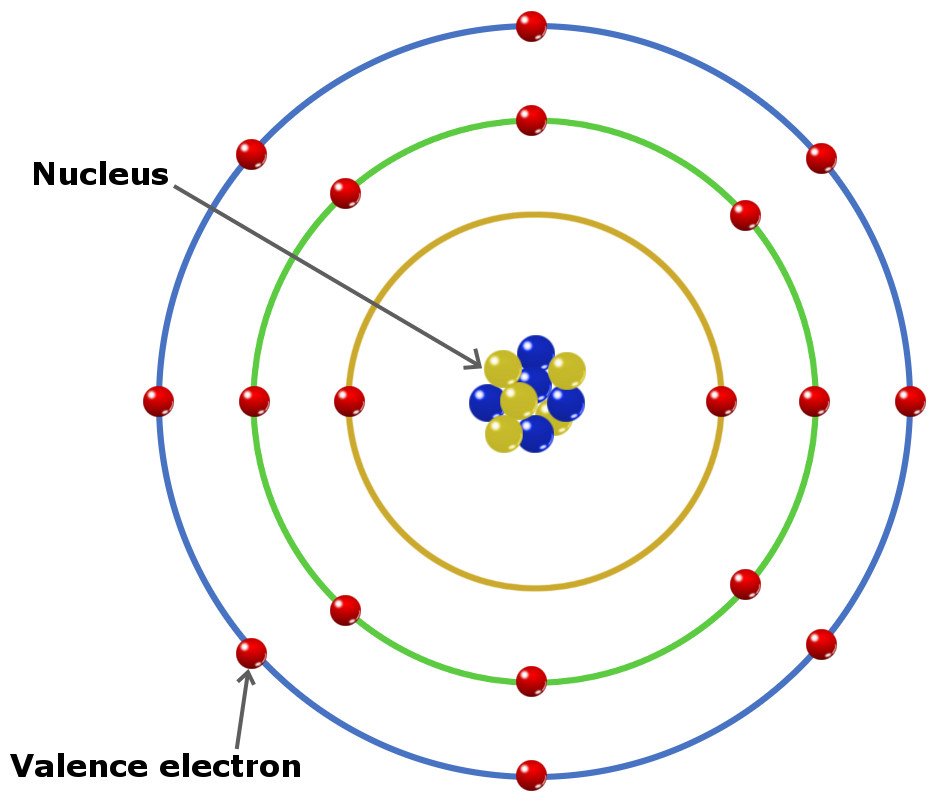
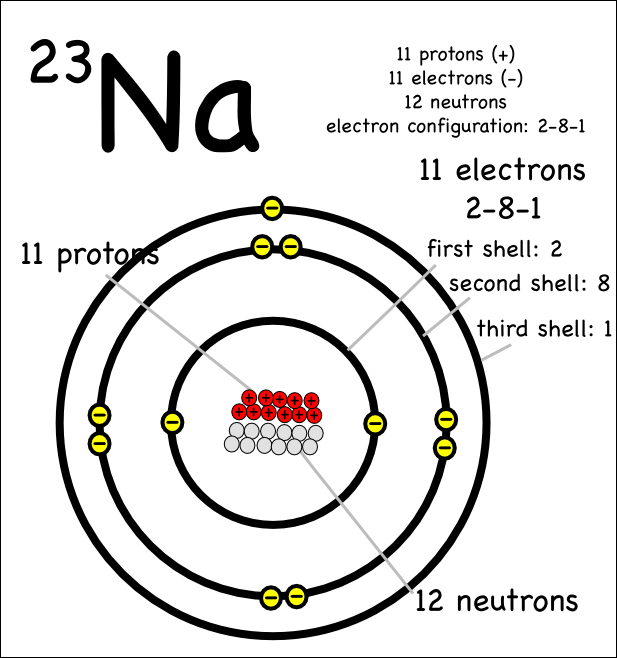
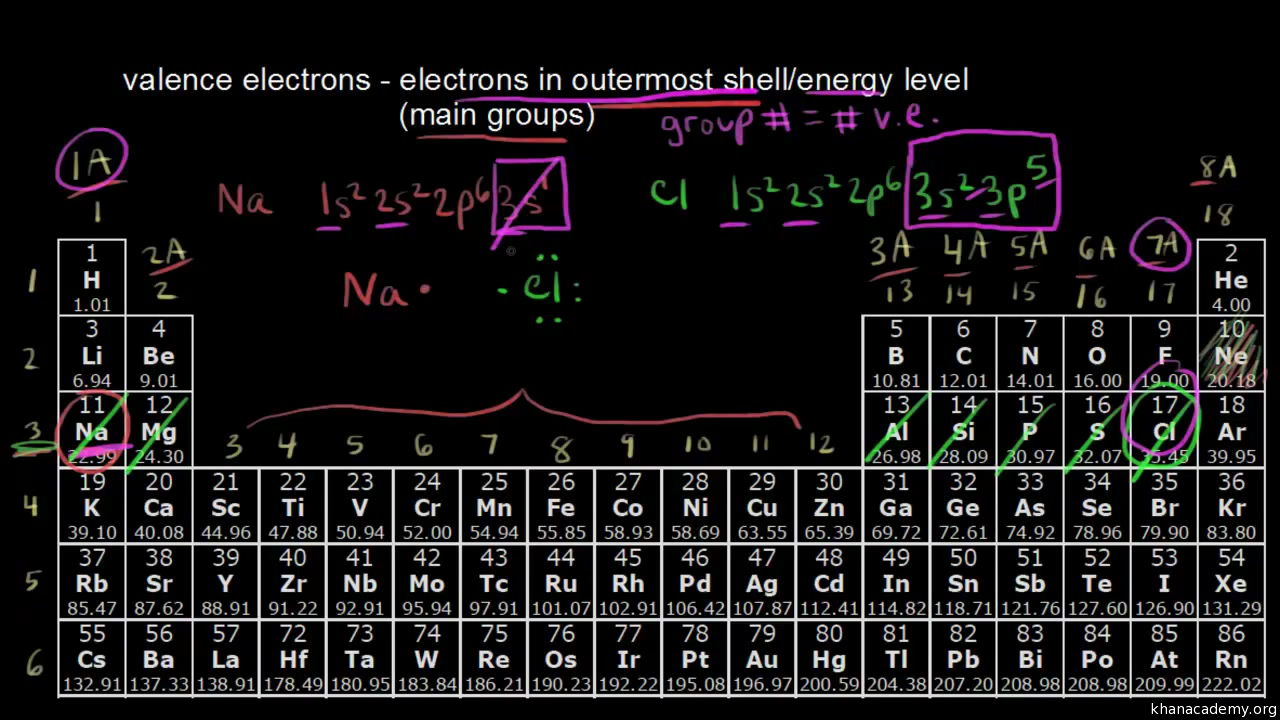
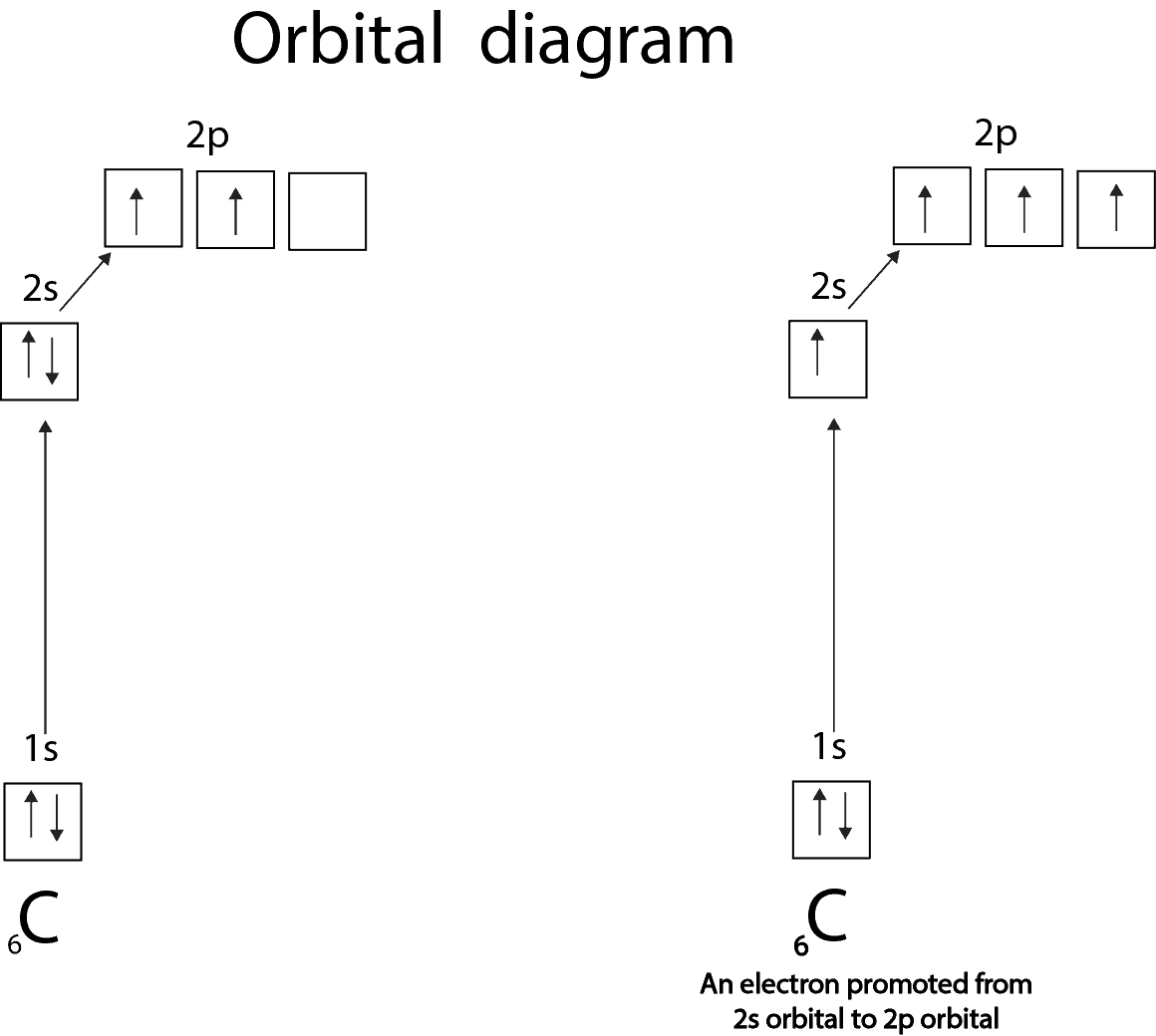
A way to find valence electrons without the periodic table is using the atomic number and drawing a diagram.
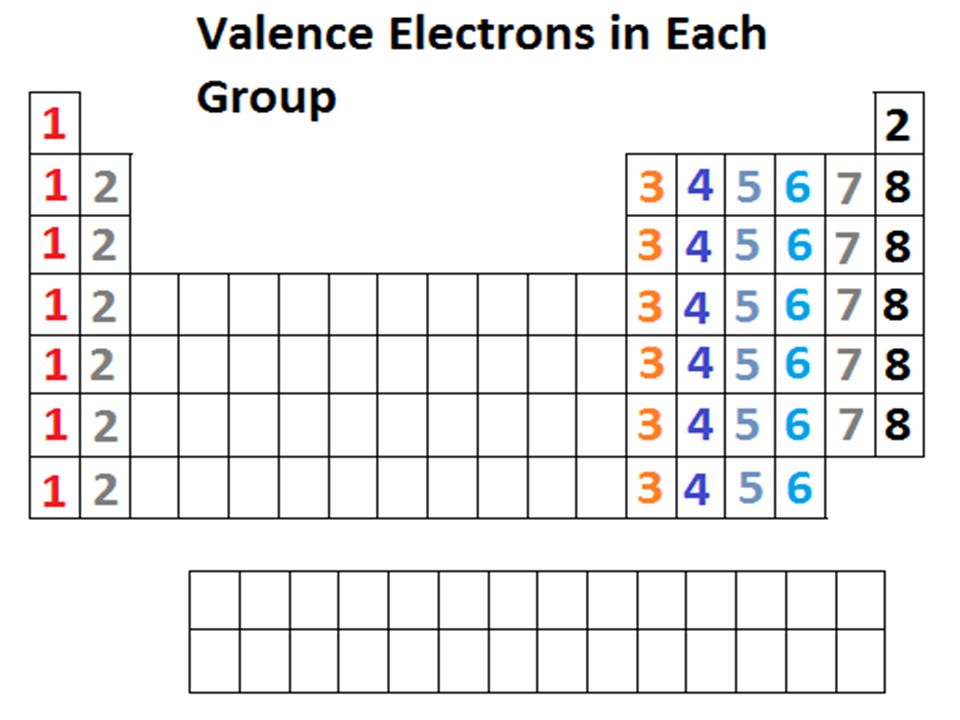
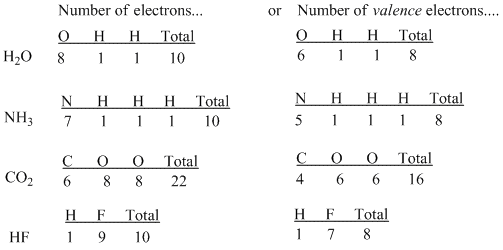
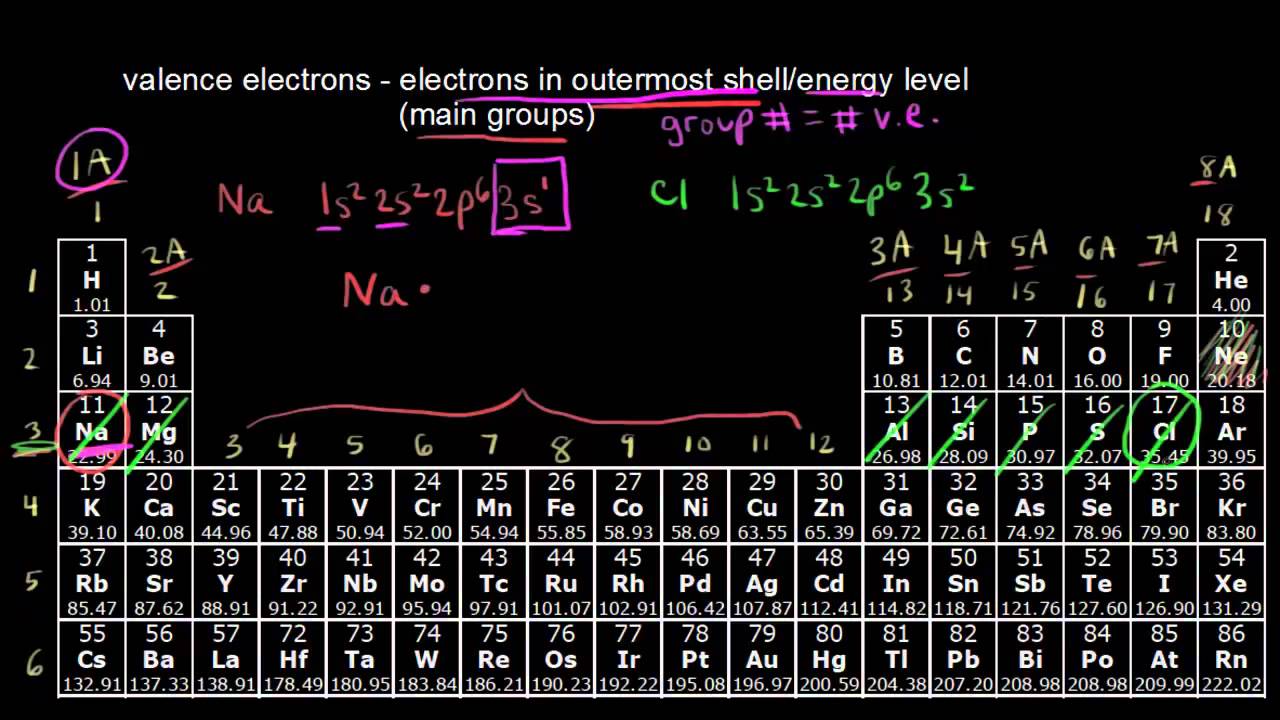
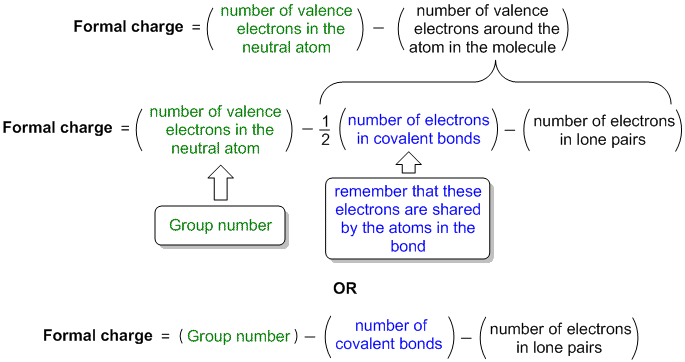
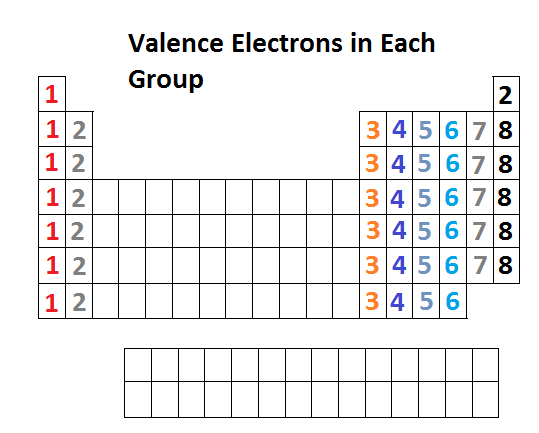
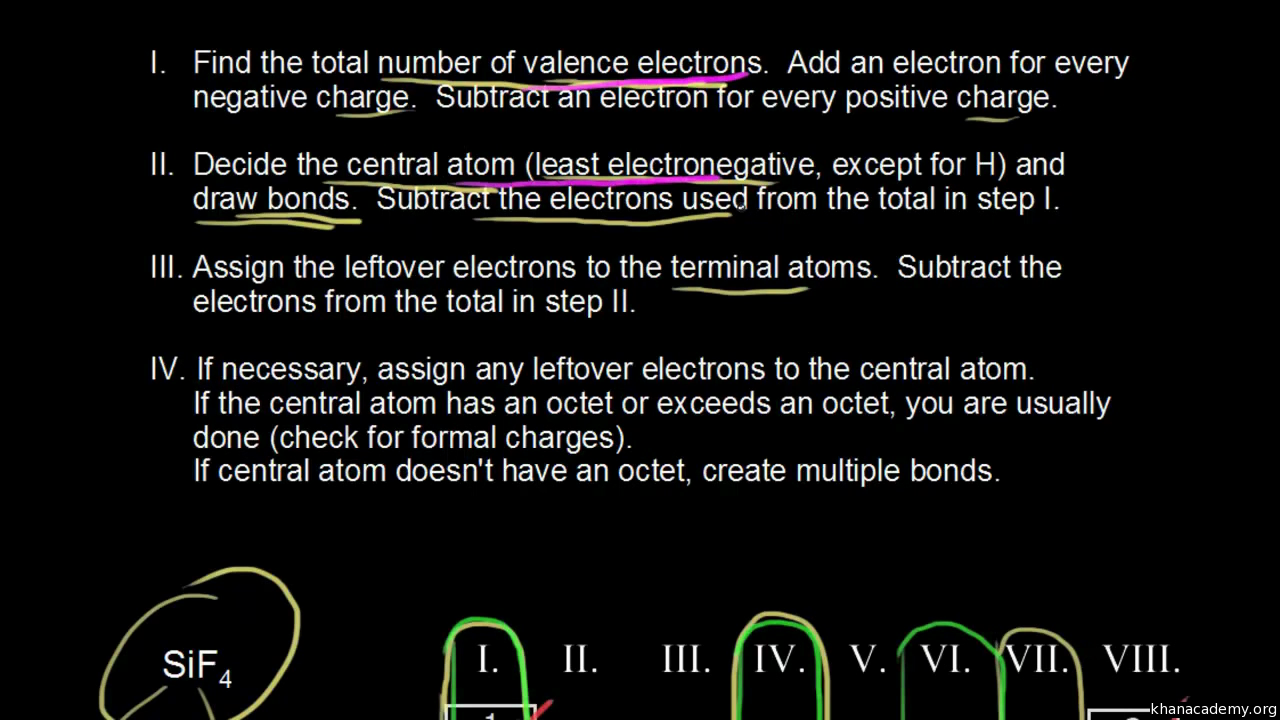
How to find number of valence electrons in a compound. Determine the total number of valence electrons in the molecule or ion. Specifically the number in the ones place. If the atom is outside this block locate its group number along the top of the table. The ones digit in the group number is the number of valence electrons.
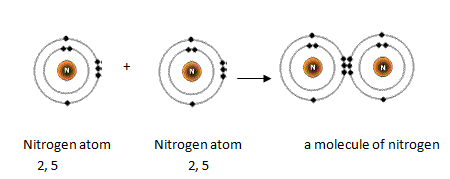
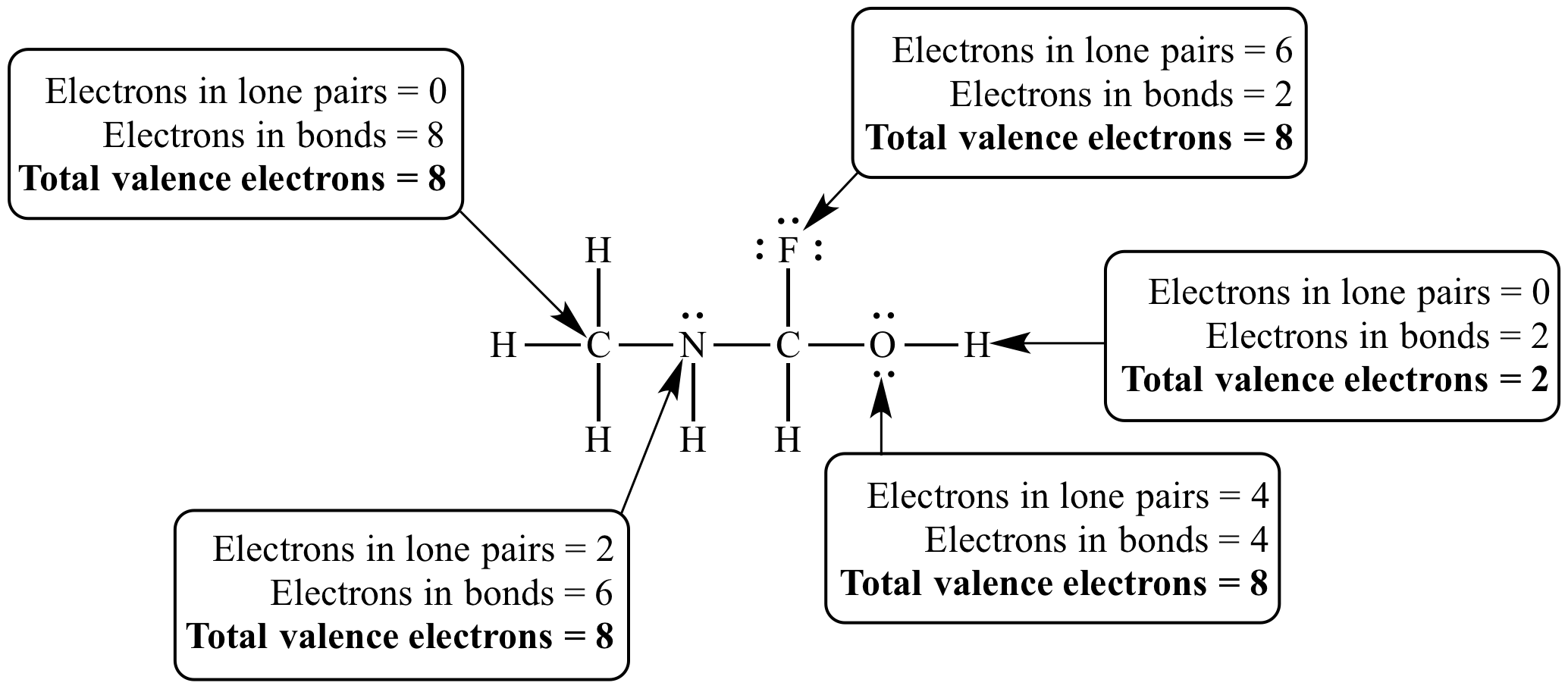
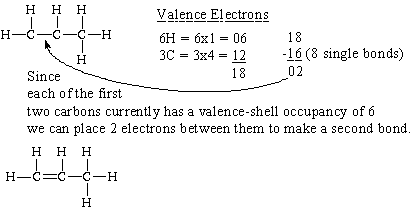
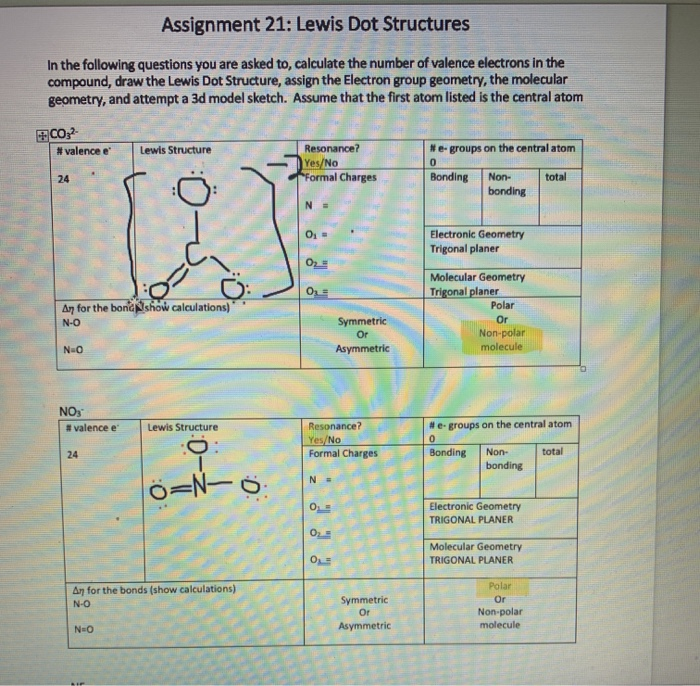
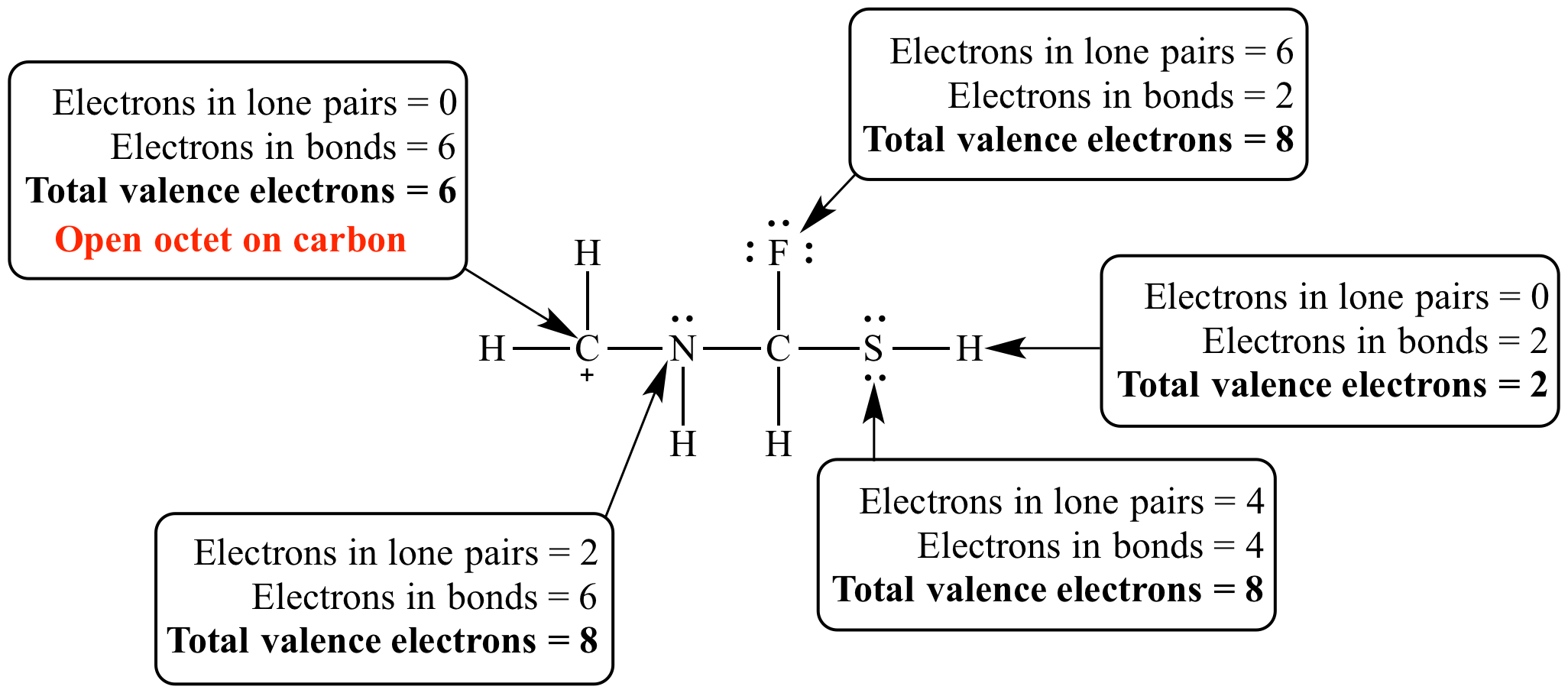
Each carbon dioxide molecule is formed from 1 catom and 2 oatoms. So the total number of valence electrons in the co molecule is 4 6 10. Lets draw it out as a simple diagram. The octet rule the tendency of an atom to seek stability by filling its outer shell with eight electrons by forming valence electron bonds can help you determine the valence of a compound once you know the maximum possible valence bonds each element can form.
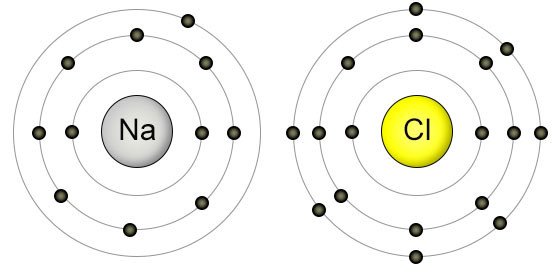
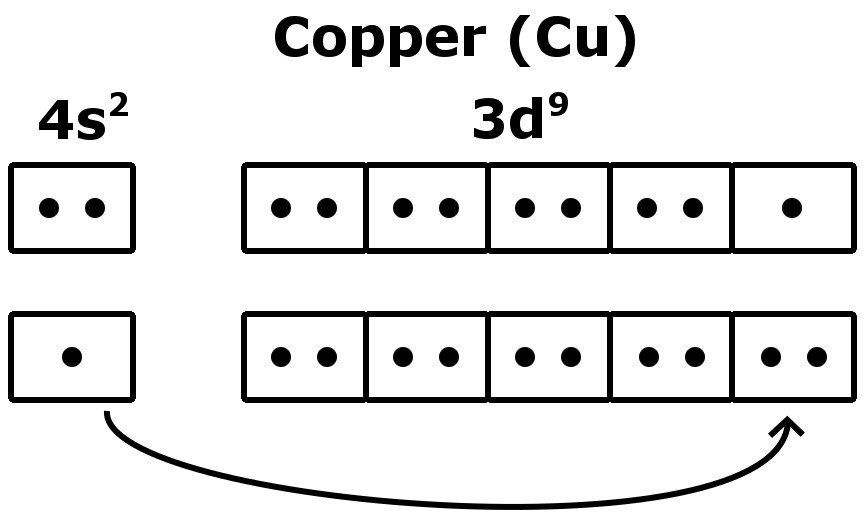
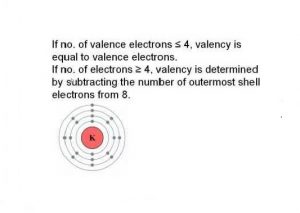
In chemistry a compounds valence number is the number of bonds formed by the electrons in the last outer shell called valence electrons of atoms to other atoms valence electrons. An elements proximity to the noble gases can help you keep track of its valence electrons in an ionic compound. The atomic number is how many protons and electrons the atom has. Here are some examples co2.
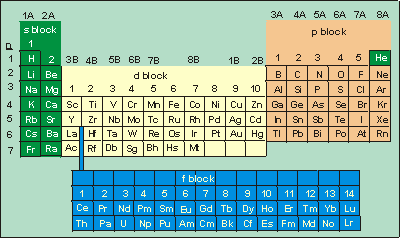
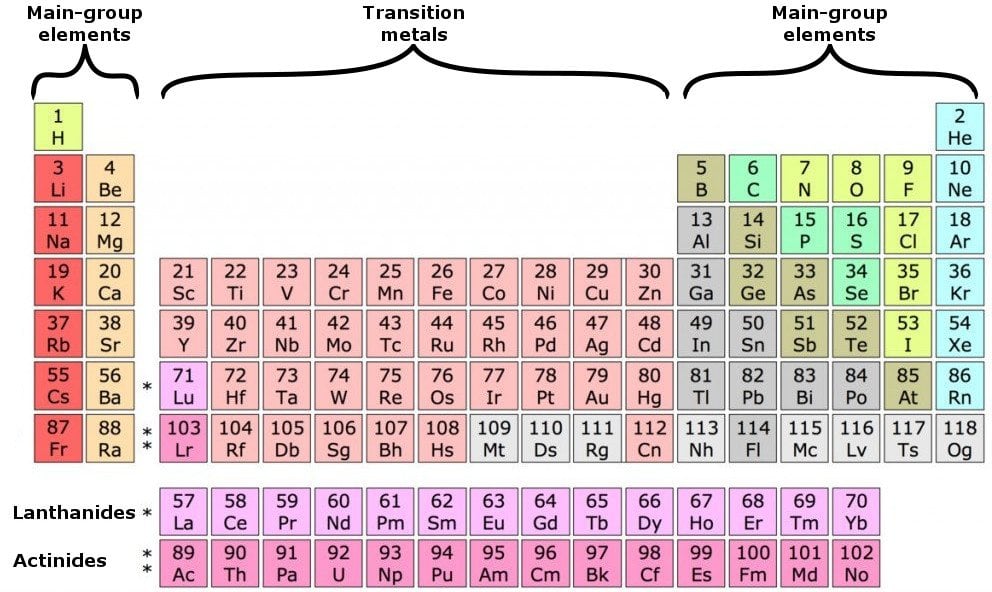
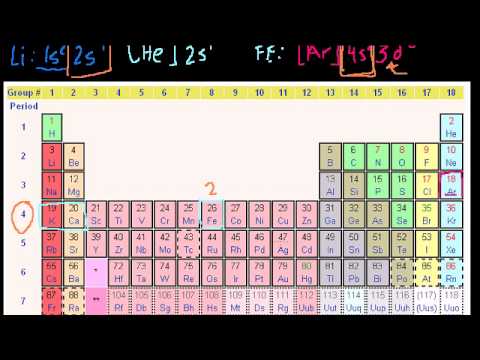
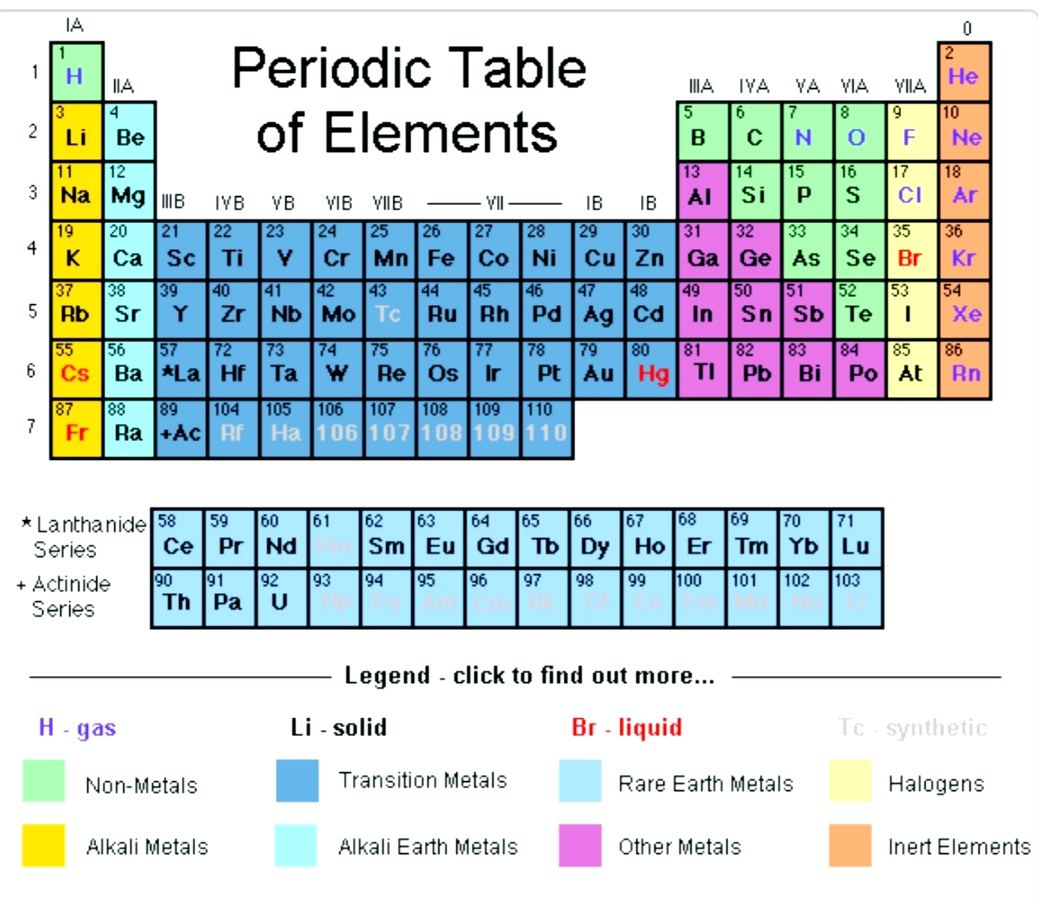
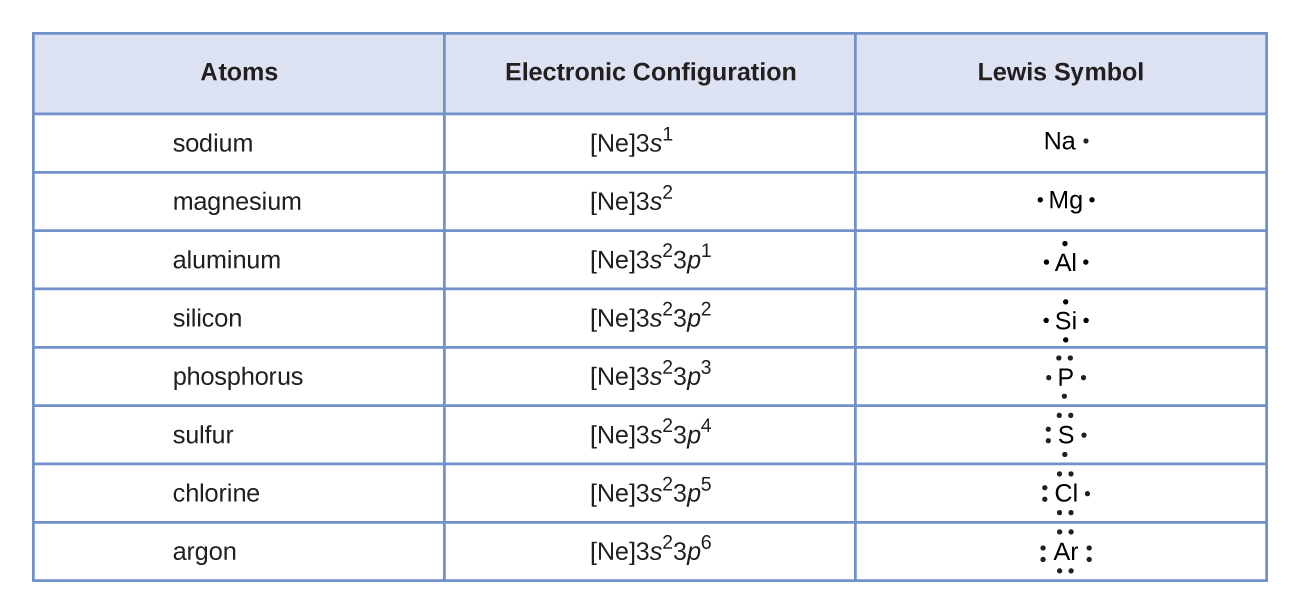
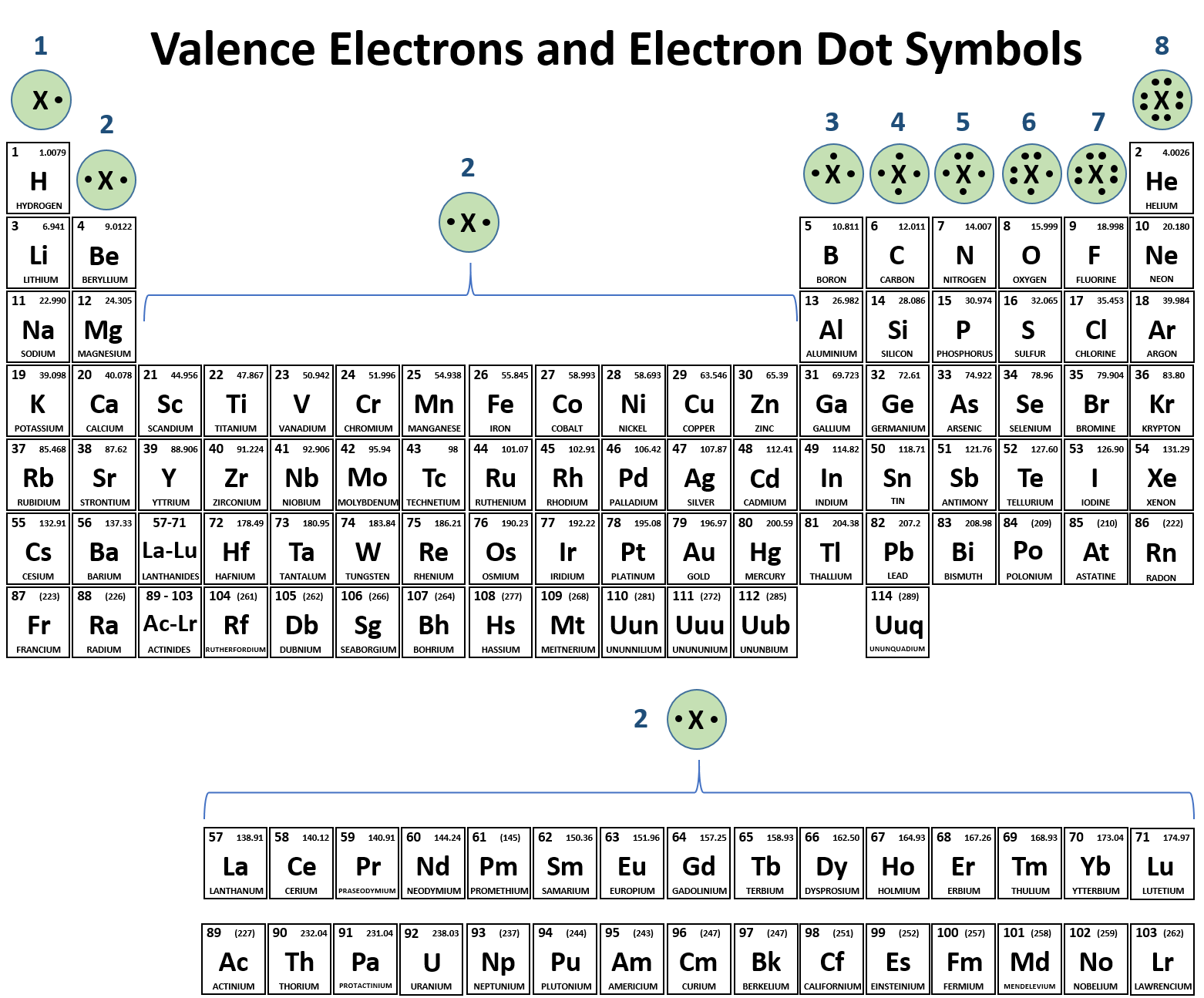
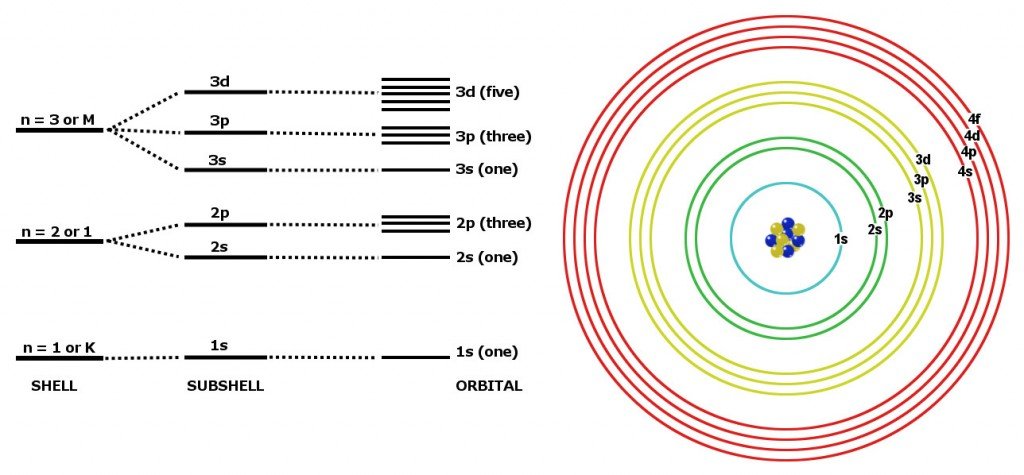
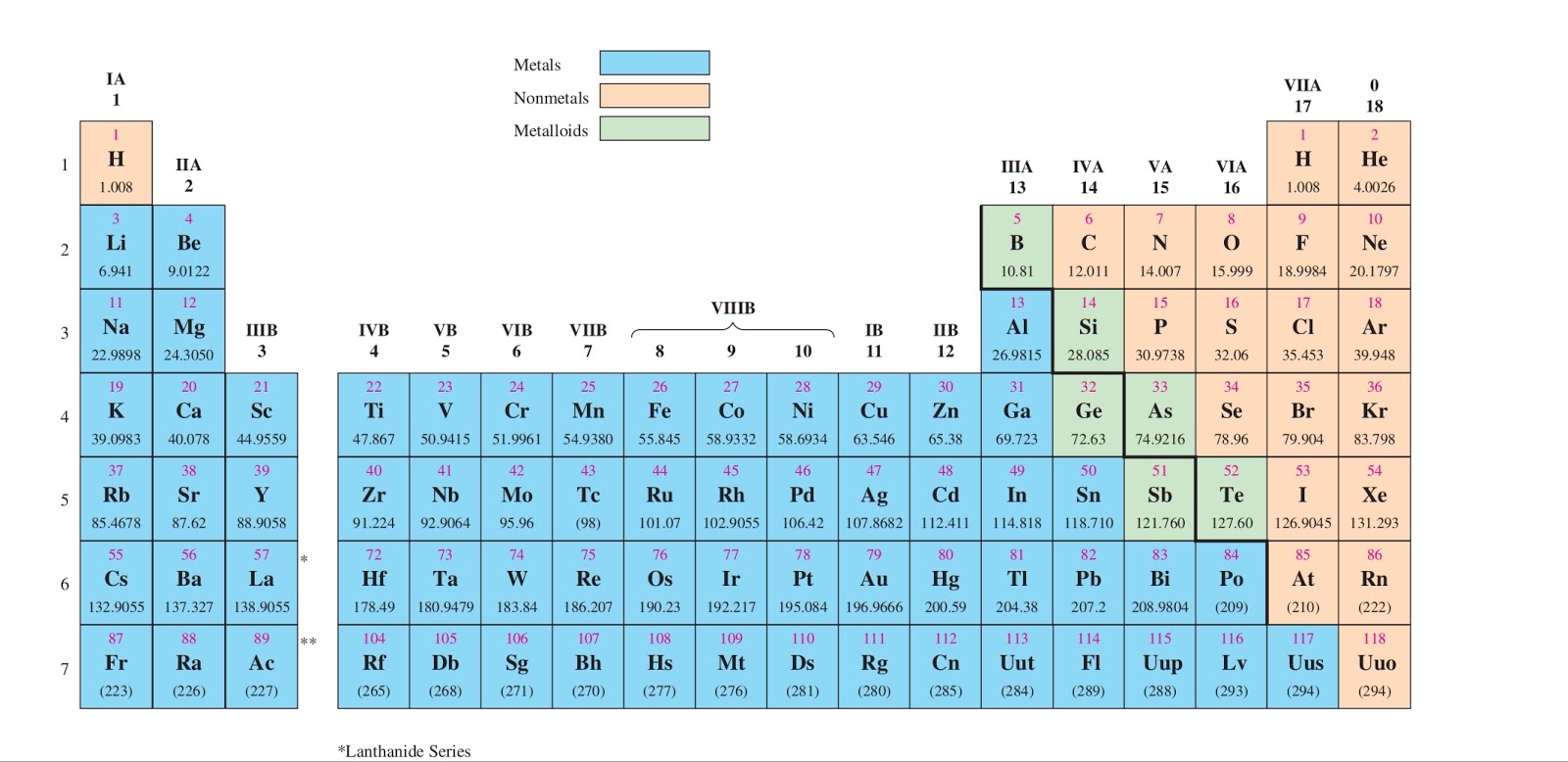
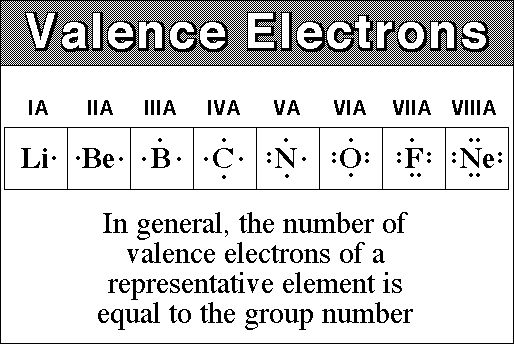
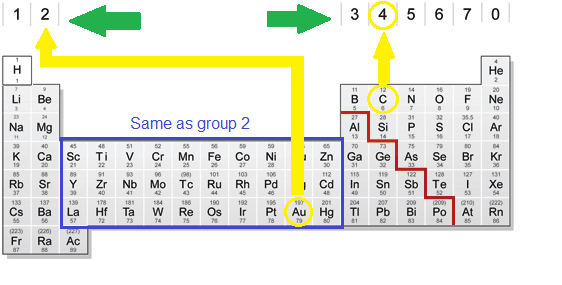
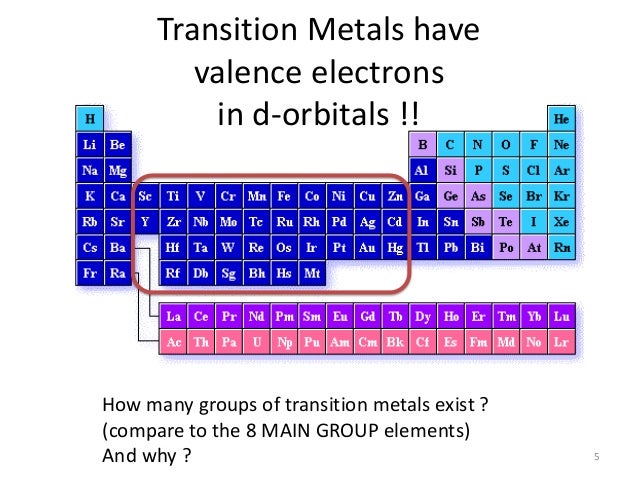
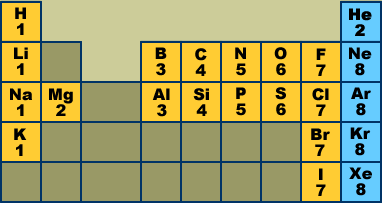
While the period number indicates the number of shells the group number indicates the number of valence electrons in the outermost shell. Recall that the number of valence electrons is indicated by the position of the element in the periodic table. To find valence electrons using a period table first see if your atom is a transitional metal which are the elements in the middle rectangle of the table. However this is only true for the main group elementsthe elements inhabiting groups 1 2 and 13 18.
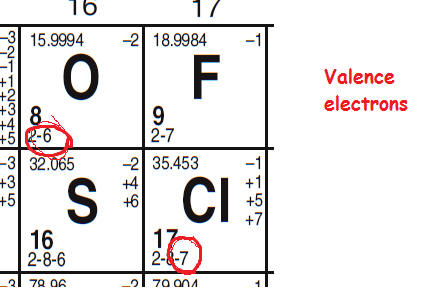
Furthermore the group number of the main group elements represents the number of valence electrons for that element in its ground state. For example a group seven element has seven electrons in its valence shell. Add together the valence electrons from each atom. Looking at the periodic table you will find o is in group 6 and so has 6 valence electrons.
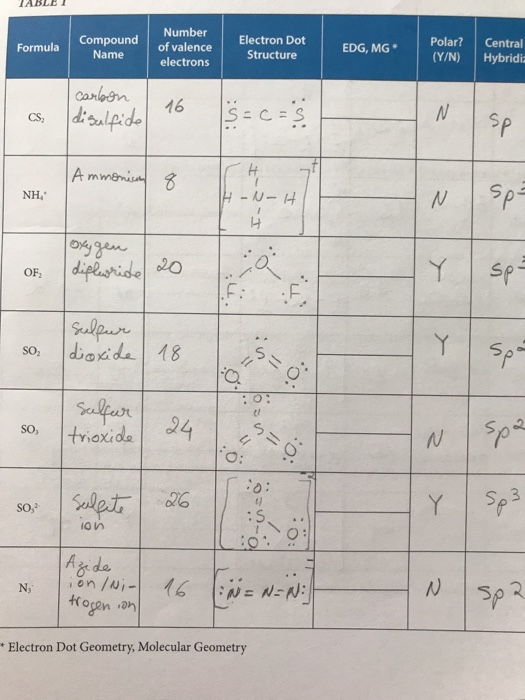
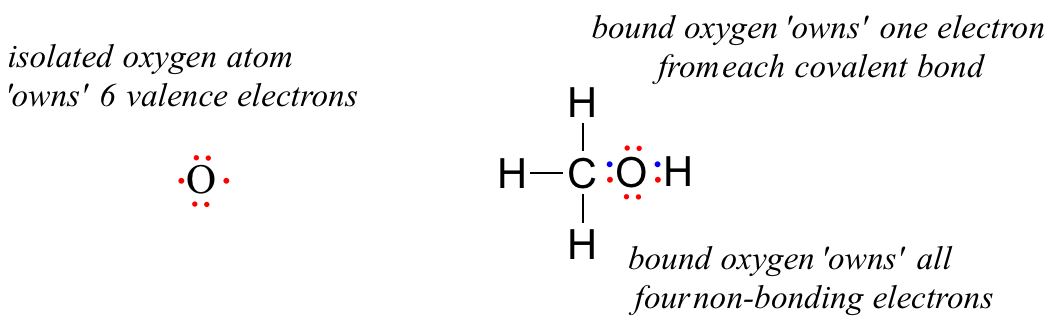
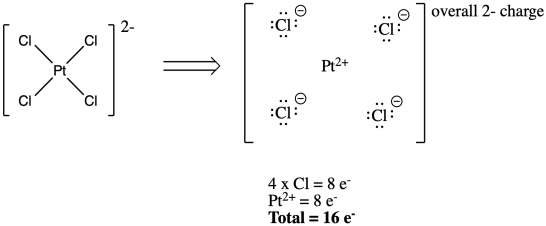
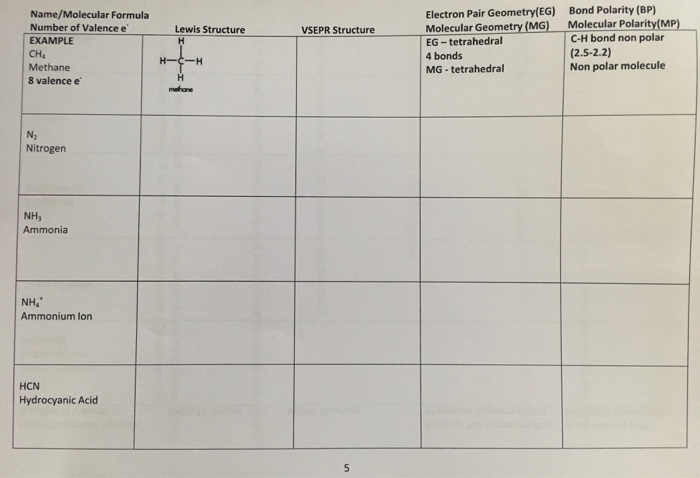
The number of valence electronsfor molecules can be calculated by adding the valence electronsof all the atomsthat form that respective molecule.




































/Lewis-dot-structure-58e5390f3df78c5162b4c3db.jpg)