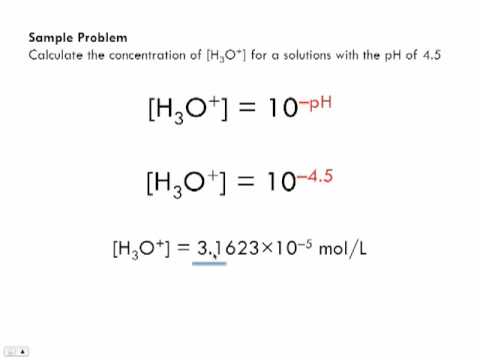
How To Find Molar Concentration Of Ions
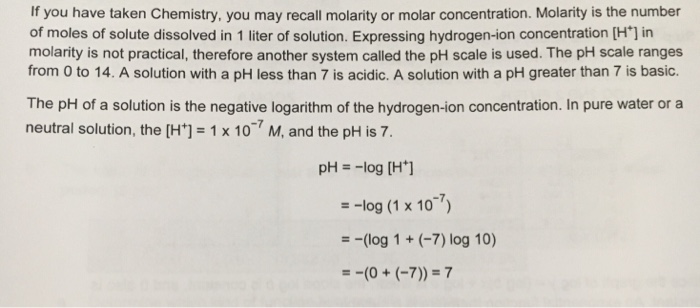
Molarity is described as the total number of moles of solute dissolved in per litre of solutionie m moll.
How to find molar concentration of ions. In many older books or articles you can find different units of molar solutions moles per liter moll. Divide the total moles of solute by the total volume of the solution in liters. Example268 g na2so4xh2o solute dissolves in water and 100 ml solution is prepared. To find the molar concentration of a solution use the concentration formula.
How to find molar concentration of ions in a solution is available in our digital library. The molar concentration formula is given by example 1. Molarity is one of the most common units of concentration. From the periodic table.
To find molar concentration of ions in a solution but end up in malicious downloads. Molar conductance at infinite dilution. Calculate the total molar conductance of nacl. Step 1 find the molecular weight or molar mass of i2 step 2 find how many moles of i2are in 508 grams i2 step 3 make sure your reaction is balanced and finde the ratio of moles of nai and i2.
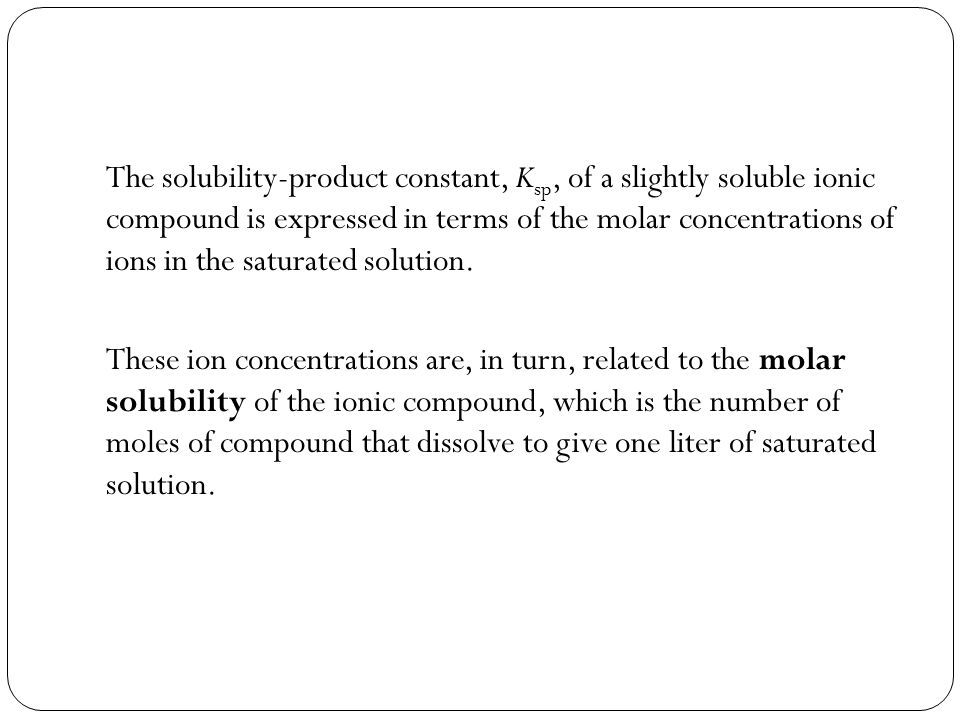
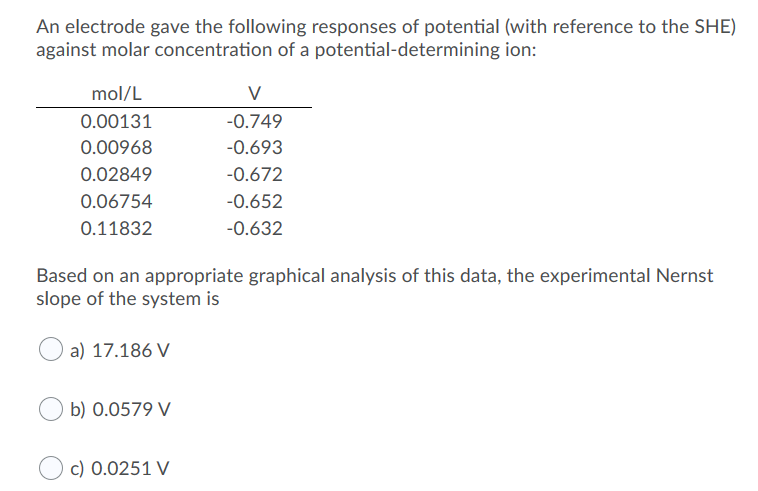
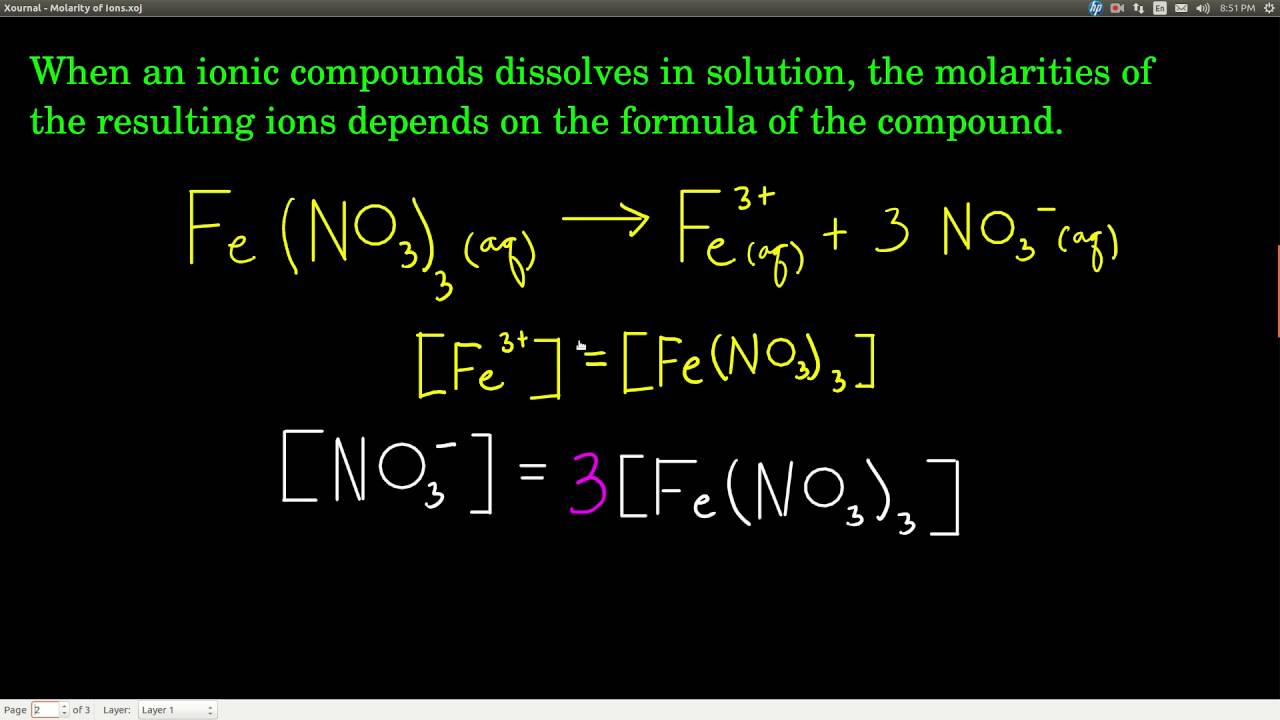
Molarity is measured in number of moles of a substance per unit volume. Do you need 2 moles nai to make one mole of i2. The molar concentration of solute is sometimes abbreviated by putting square brackets around the chemical formula of the solute eg the concentration of hydroxide anions can be written as oh. Molar conductance values at infinite dilution of na and cl ions are 5112 10 4 sm 2 mol 1 and 7354 10 4 sm 2 mol 1 respectively.
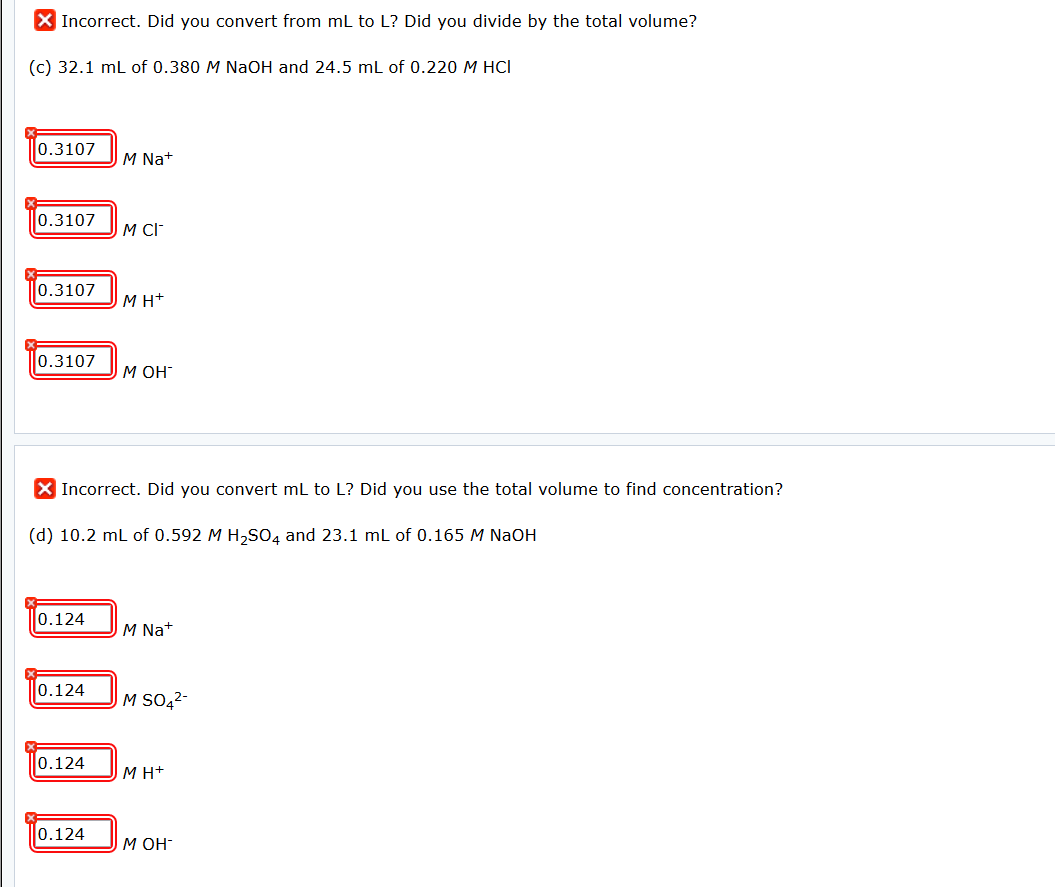
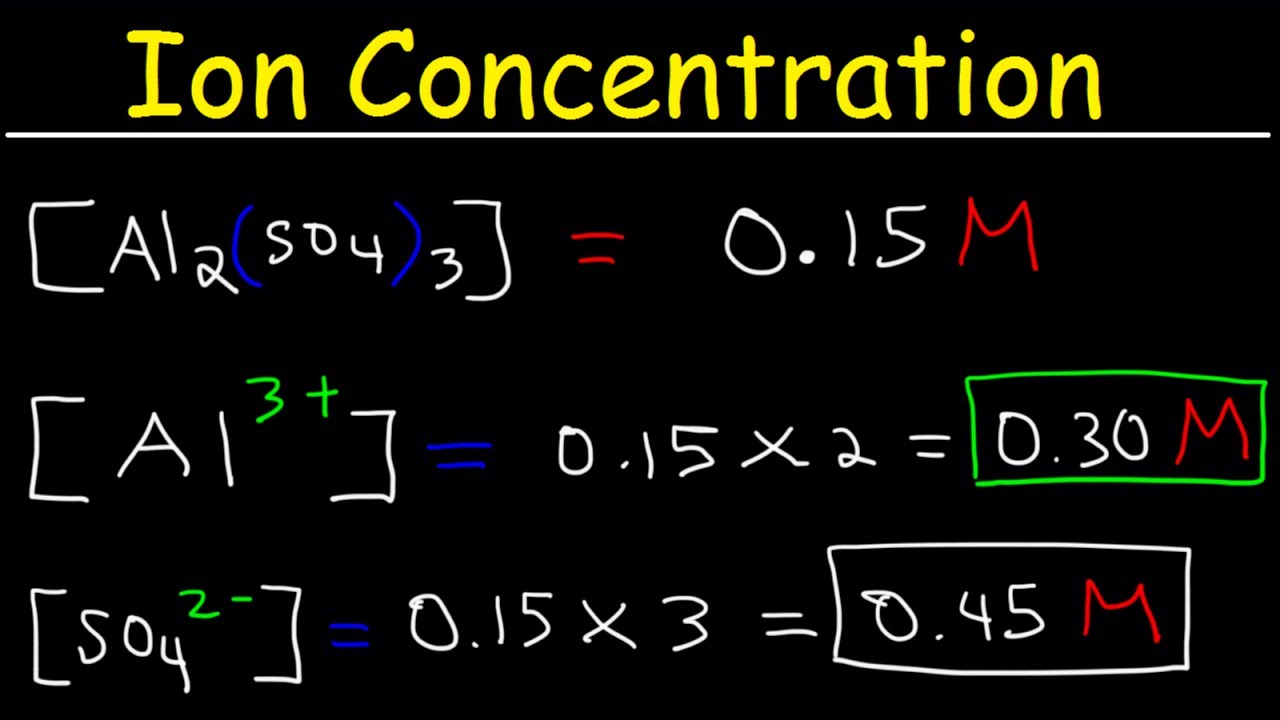
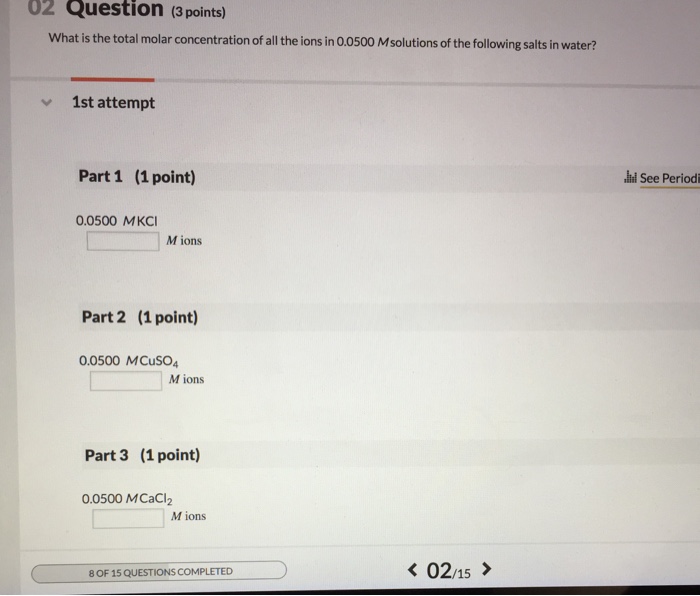
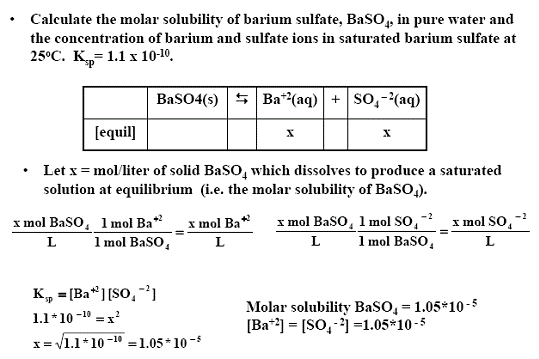
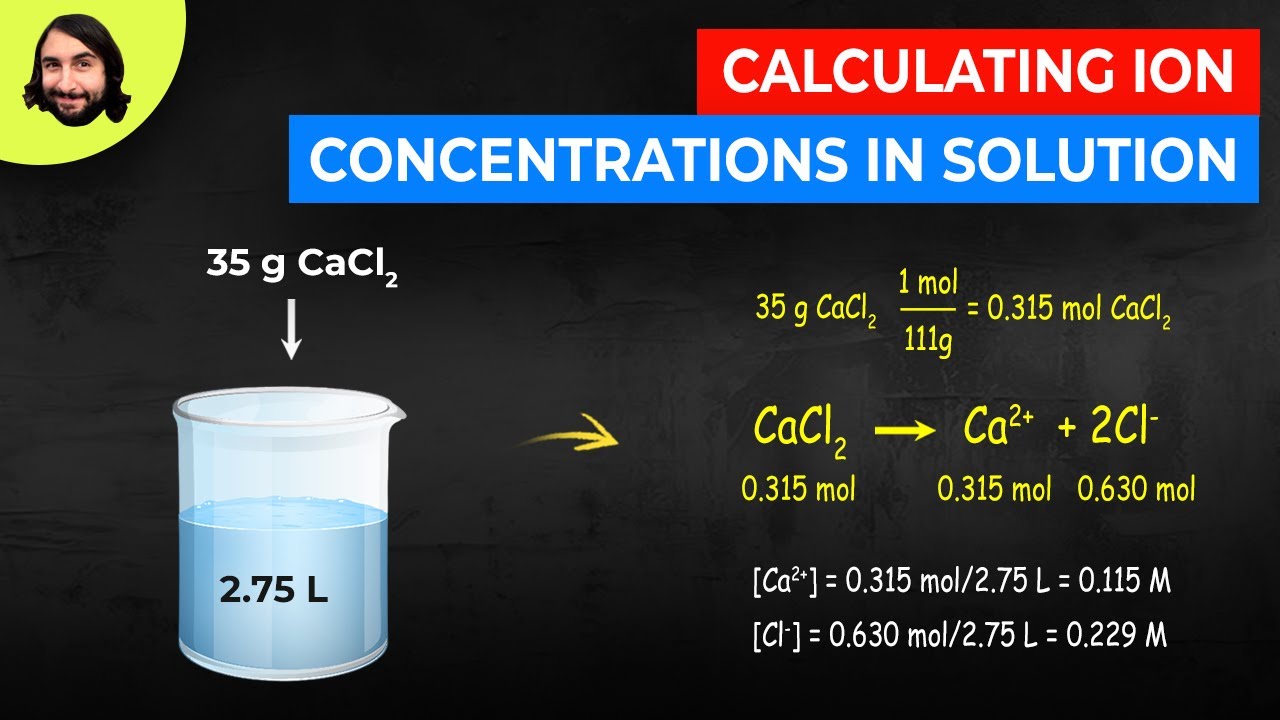
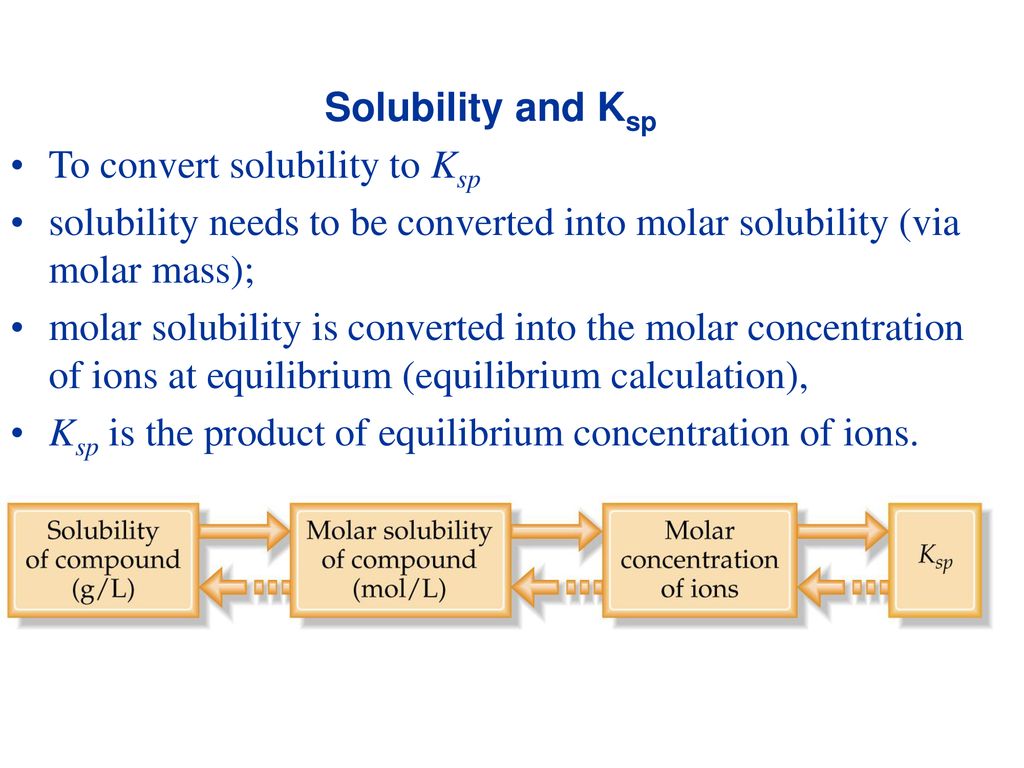
Atomic mass of cu 6355atomic mass of cl 3545atomic mass of cucl2 16355 23545atomic mass of cucl2 6355 709. All moles measurements are applied to determine the volume of moles in the solution that is the molar concentration. Rather than enjoying a good book with a cup of tea in the afternoon instead they cope with some harmful virus inside their laptop. To find the molarity of the ions first determine the molarity of the solute and the ion to solute ratio.
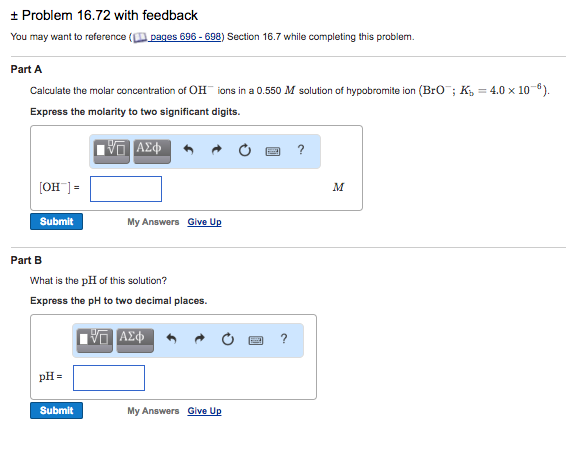
Molar conductivity of the kcl solution is 124 cm2 mol1. Find the molarity of the solute.

















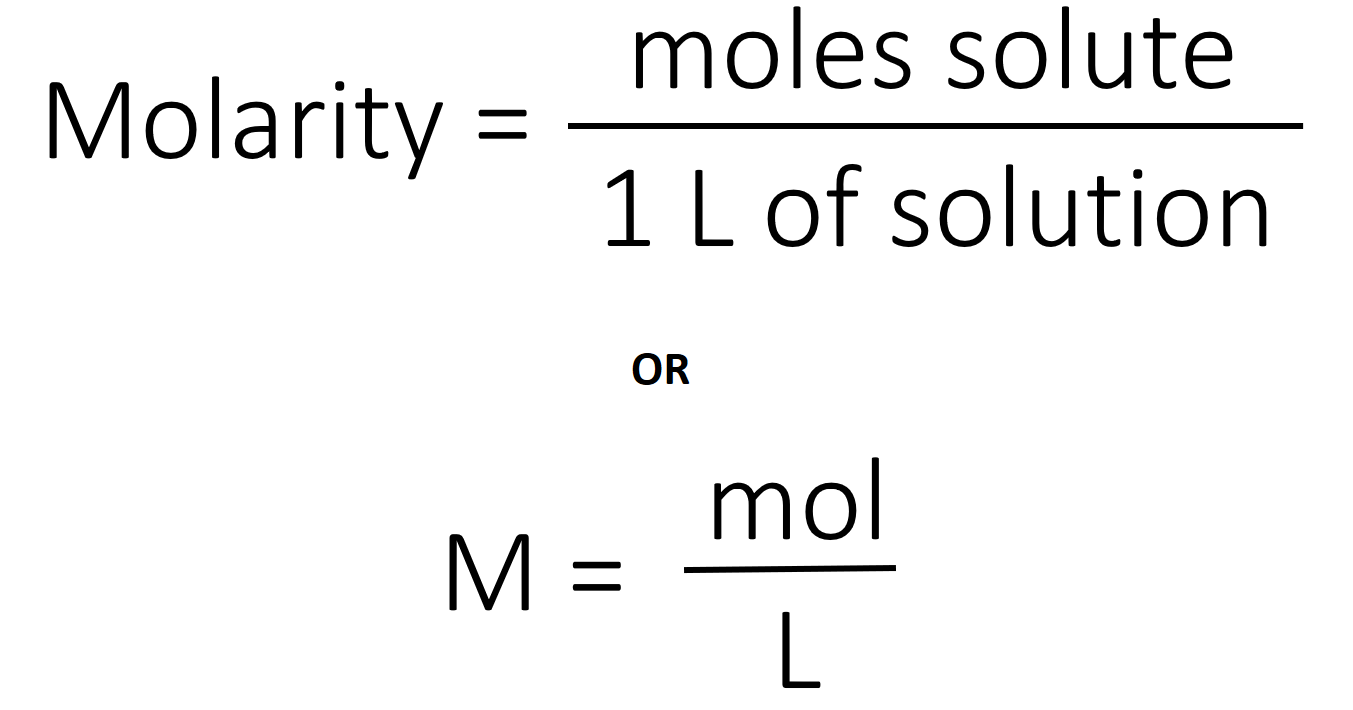










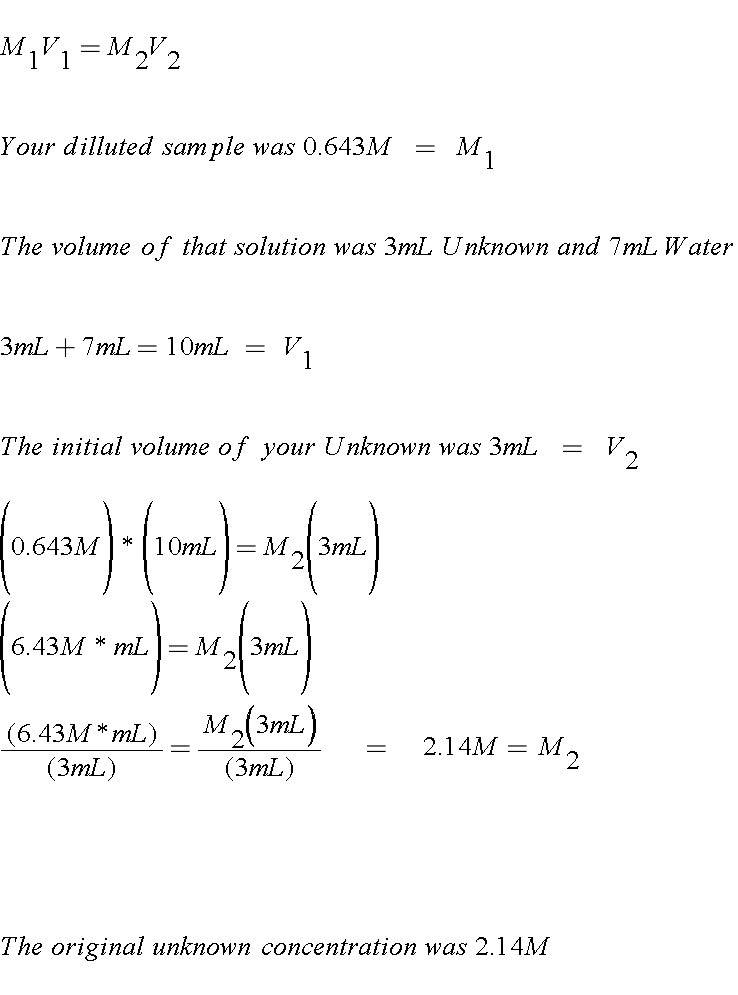
/how-to-calculate-ph-quick-review-606089_final-165915b0177b4f6e82843f25097f51df.png)








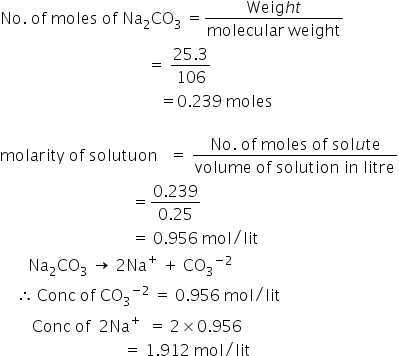




/how-to-calculate-normality-609580final2-0d5efa5a961f4fa0a7efc780921faee1.png)

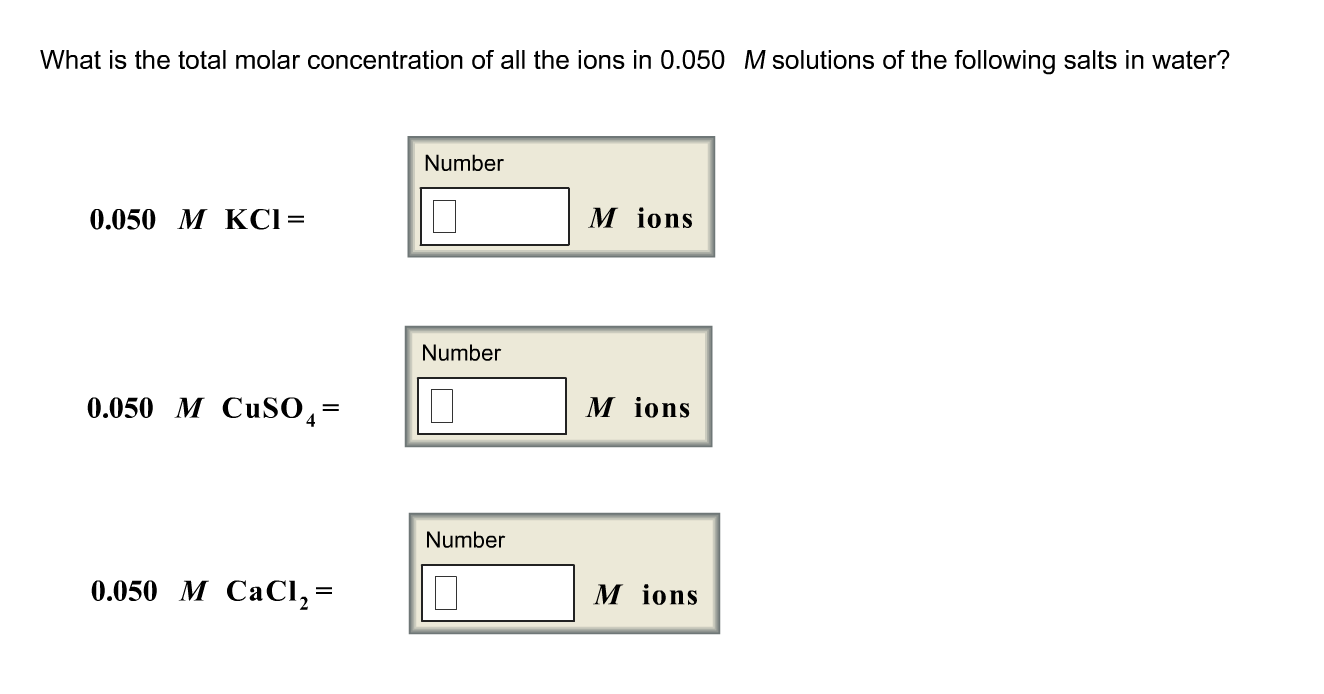
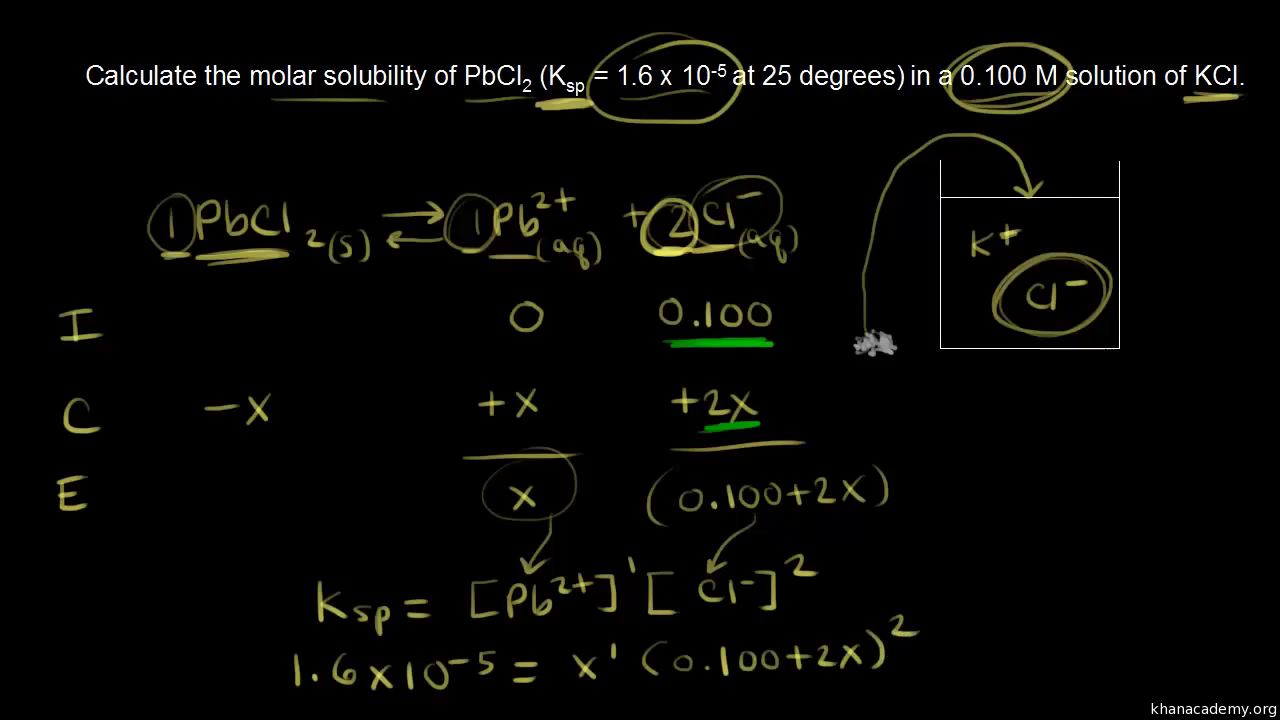


:max_bytes(150000):strip_icc()/erlenmeyer-flask-containing-a-solution-of-potassium-permanganate--kmno4---various-flasks-with-transition-metal-salts--dry-chemicals-and-solutions-in-background-702545749-5c05a3ffc9e77c0001dfc39c.jpg)
:max_bytes(150000):strip_icc()/test-tube-chemicals-56a12dc25f9b58b7d0bcd0db.jpg)