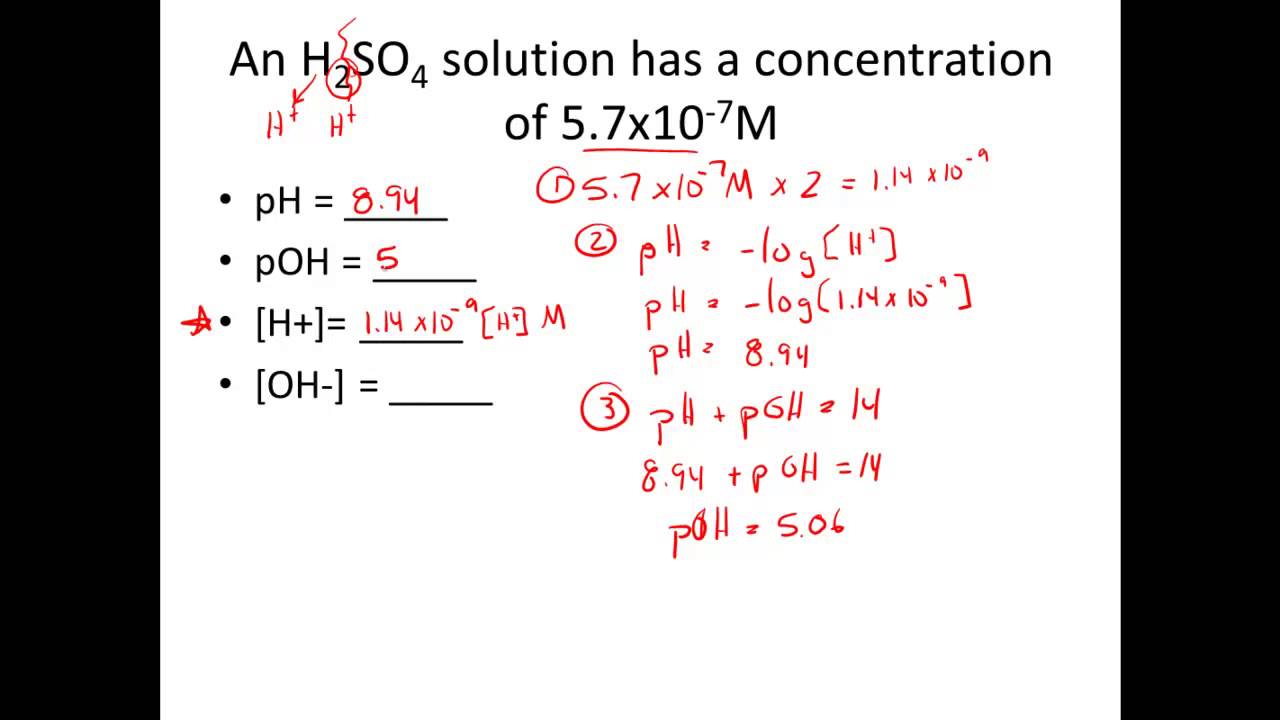
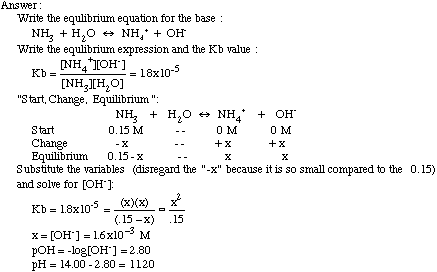How To Find Molar Concentration From Ph
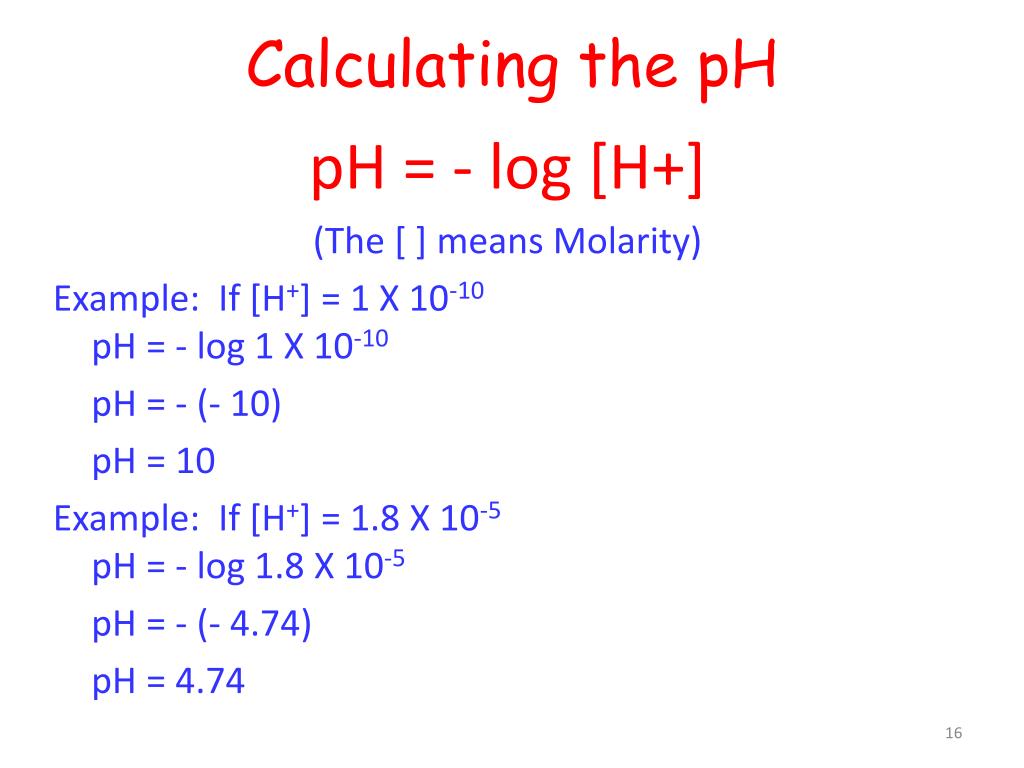
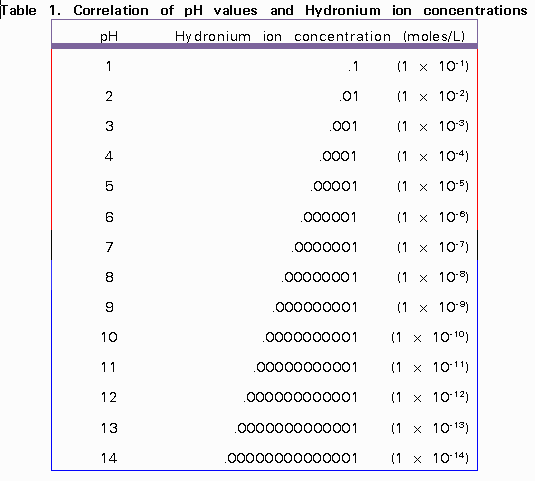
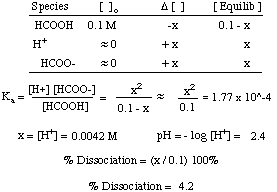
Ph log 63 10 5 42.

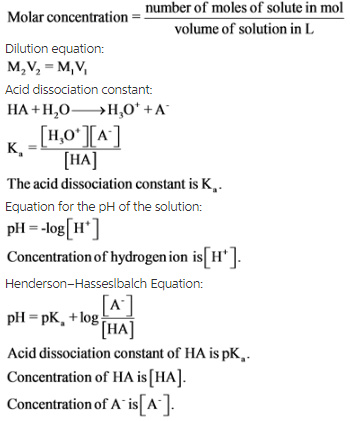
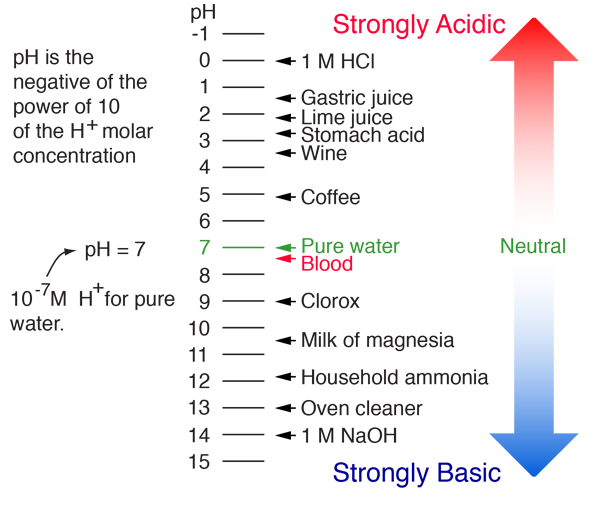
How to find molar concentration from ph. In other words ph is the negative log of the molar hydrogen ion concentration or the molar hydrogen ion concentration equals 10 to the power of the negative ph value. The definition of molarity means that you can find the molarity of a solution if you know the total number of moles of the solute and the total volume of the solution. Find ph from h3o. Its easy to do this calculation on any scientific calculatorbecause more often than not these have a log button.
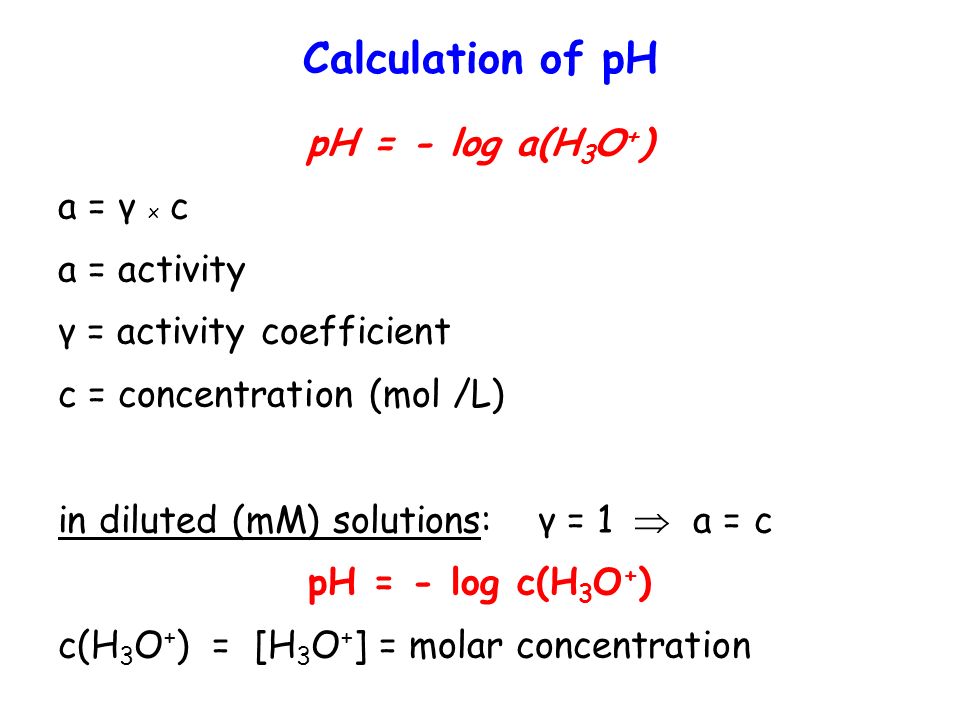
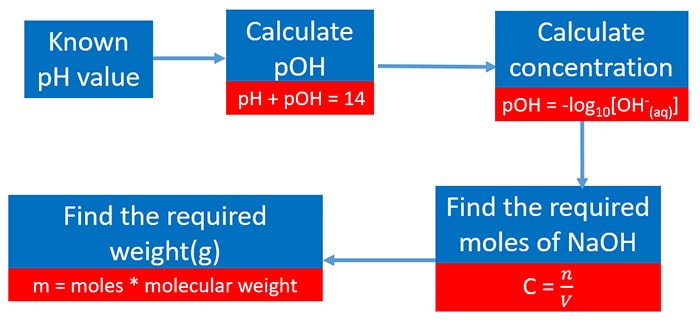
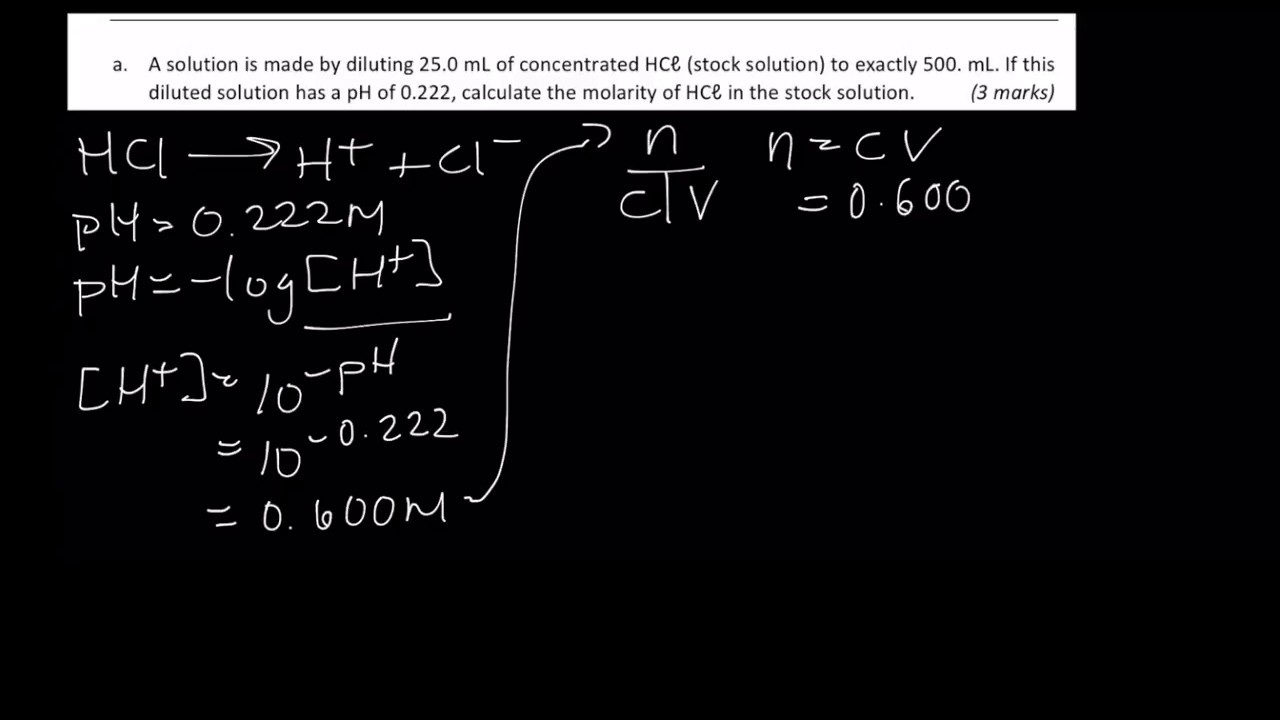
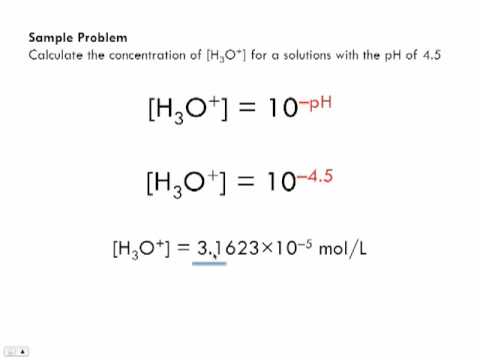
Calculate concentration of solution of known ph. Ph log h3o the ph of a solution is equal to the negative logarithm of the hydronium ion h3o concentration. Calculate the naoh weight required to prepare 500ml of naoh solution of ph13. The hydrogen ion concentration of a solution with ph 55 is 3210 6 m.
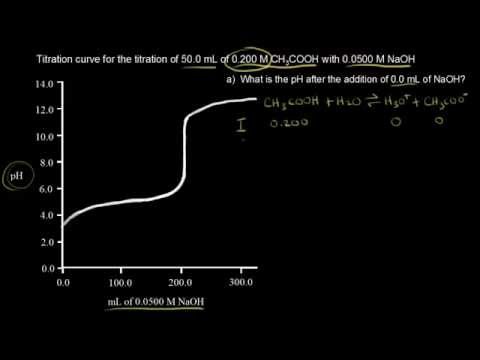
Room temperature is 25 0 c. The higher the k a for a particular acid the stronger the acid it is. This is the concentration or molarity. More ph problems calculate hydrogen ion concentration from ph 001 duration.
Calculate poh from ph by using the ph poh 14 at 25 0 c then you can calculate the concentration of naoh solution by poh log 10 oh aq. Ph log 00025 260 260 top calculating the hydronium ion concentration from ph. A ph of 55 tells you about acidity and you can calculate the molarity if you need it. For example if the original volume of the analyte was 500 ml divide by 1000 ml per l to obtain 05 l.
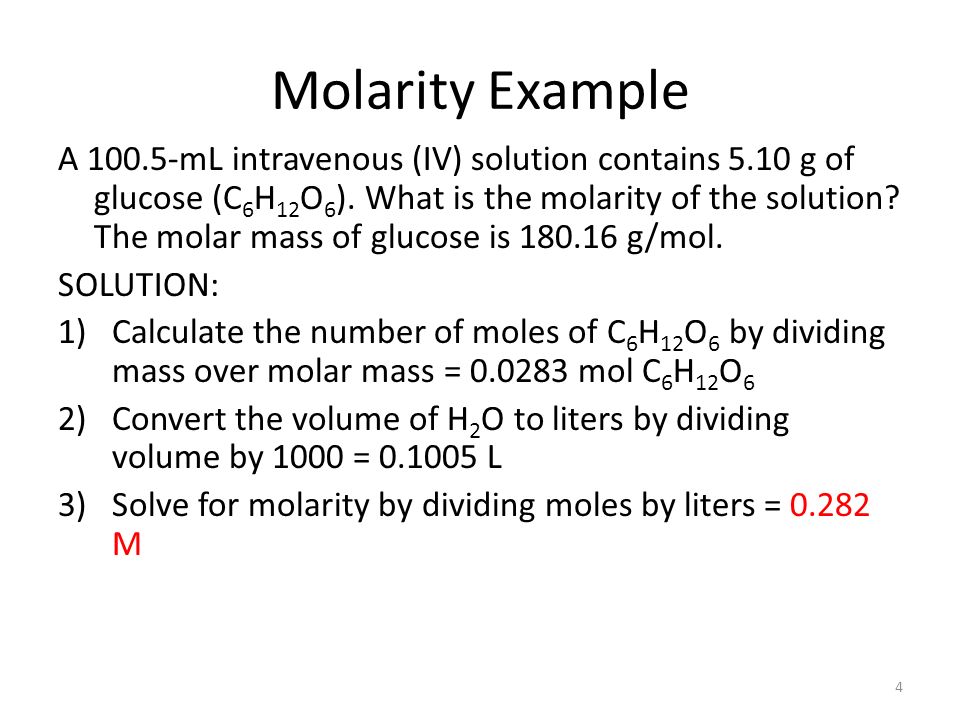
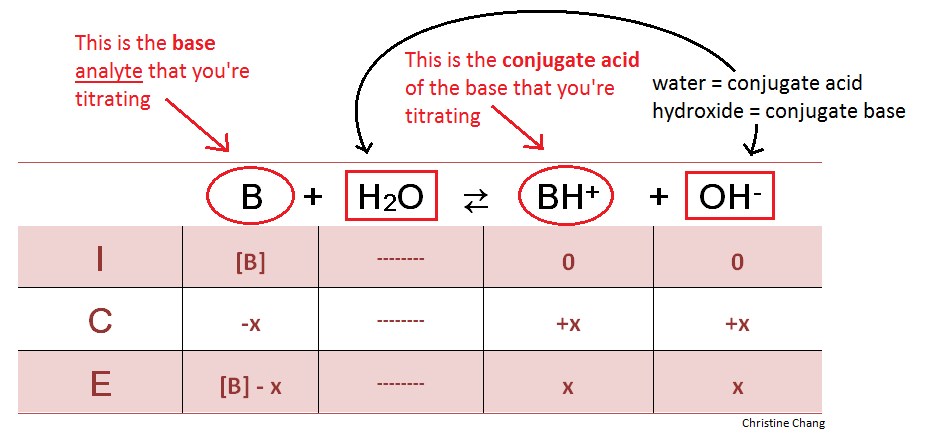
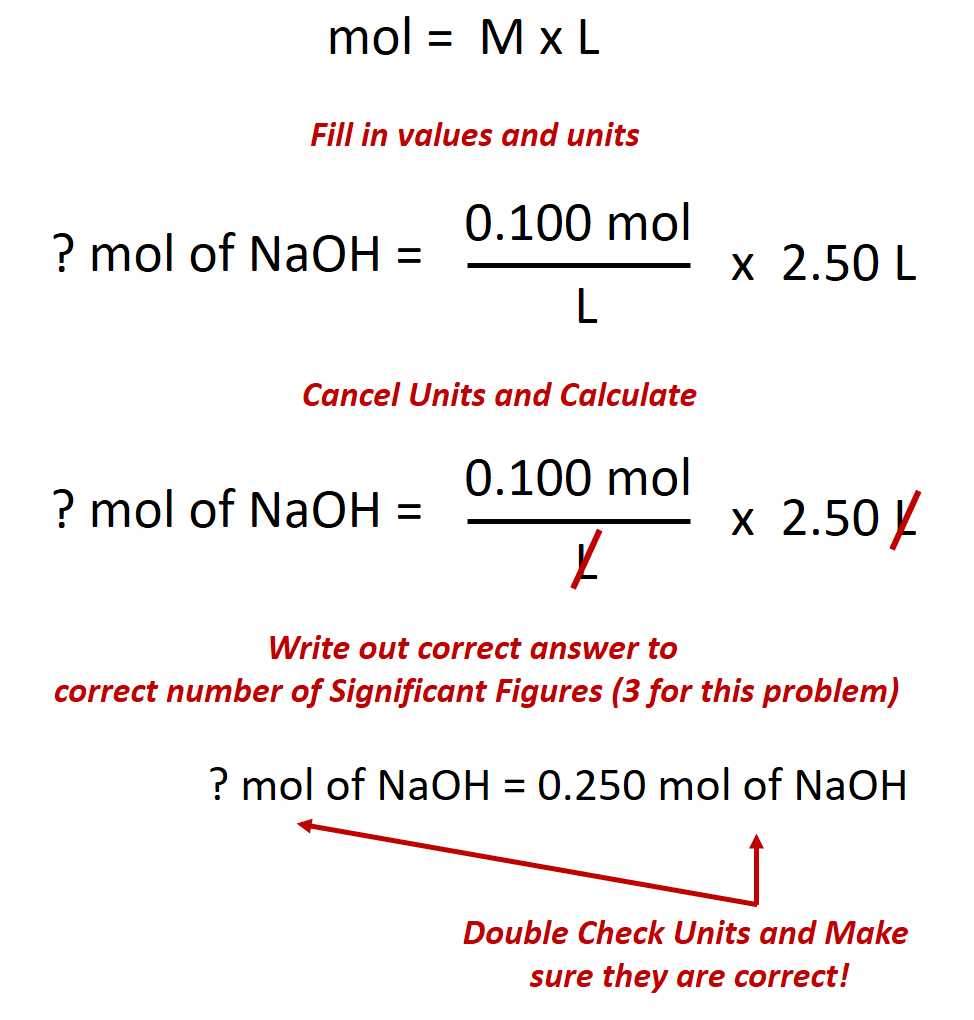
So in order to calculate the concentration of a solution in molarity you need to divide moles of solute by total volume. The hydronium ion concentration can be found from the ph by the reverse of the mathematical operation employed to find the ph. Professor heaths chemistry channel 12121 views. Divide 001 moles of analyte by 05 l to obtain 002 moles per liter.
Divide the number of moles of analyte present by the original volume of the analyte. The hydronium ion concentration is 00025 m. In a 10 l sample of 01 m hydrochloric acid hcl the concentration of hydronium ions is 1 10 1.

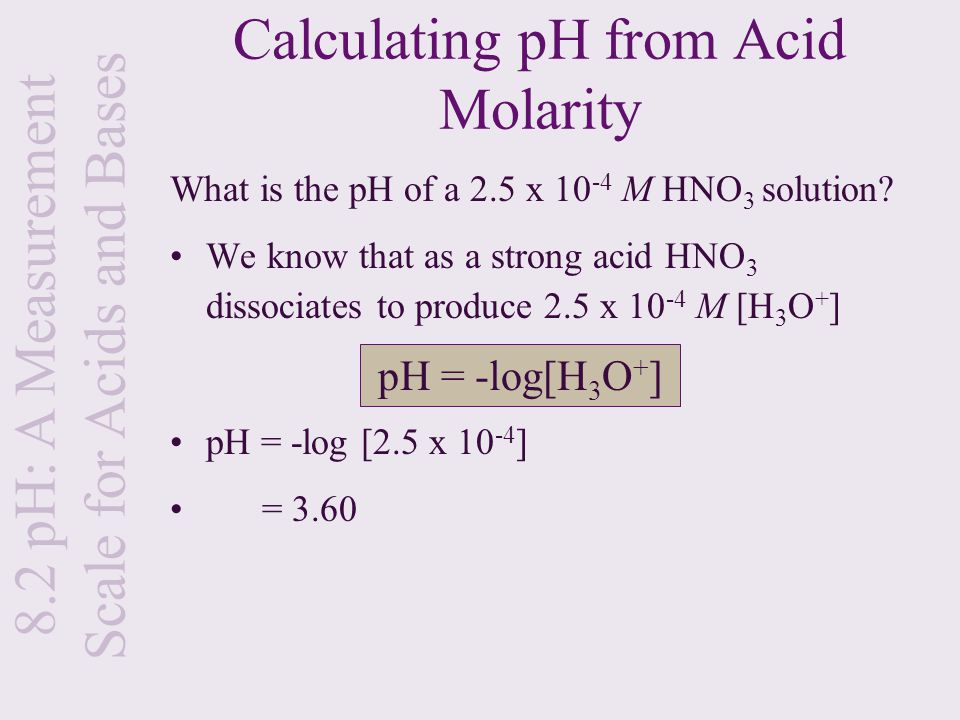
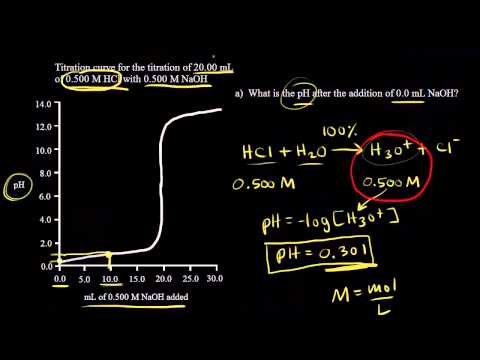








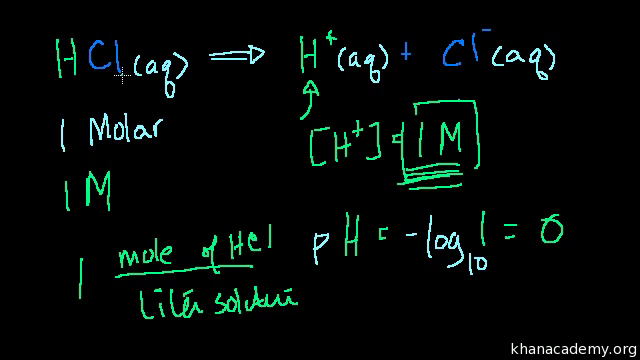







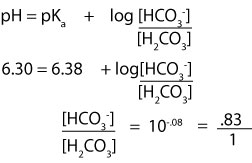



/how-to-calculate-normality-609580final2-0d5efa5a961f4fa0a7efc780921faee1.png)

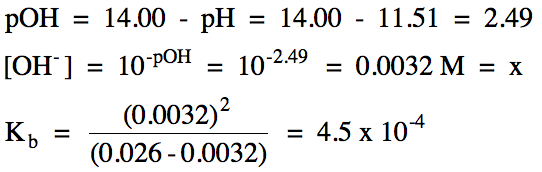
















/how-to-calculate-ph-quick-review-606089_final-165915b0177b4f6e82843f25097f51df.png)