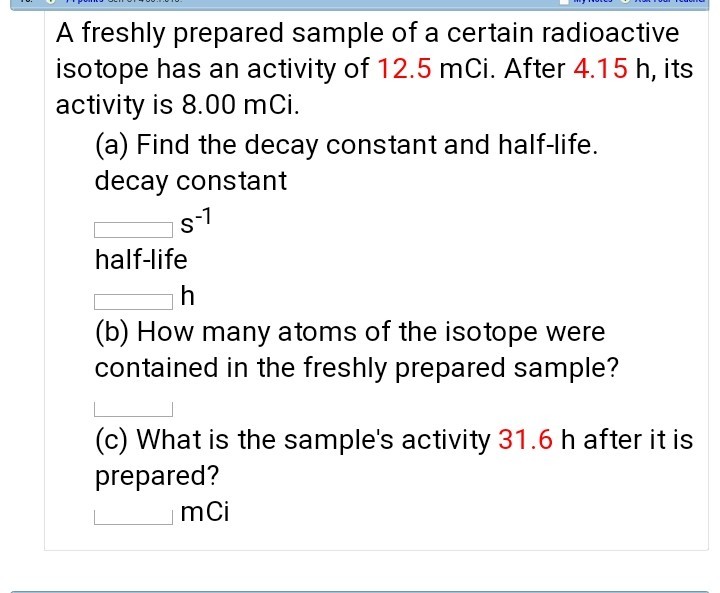
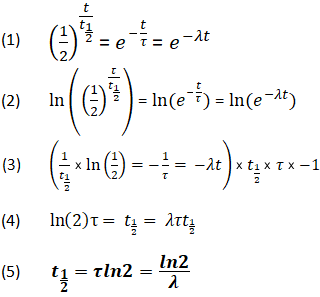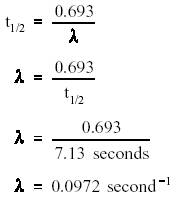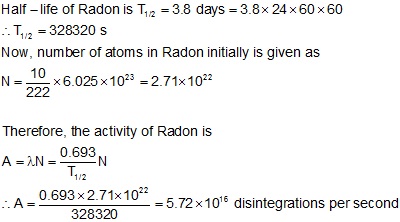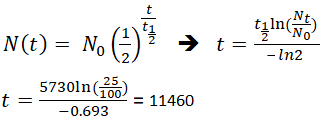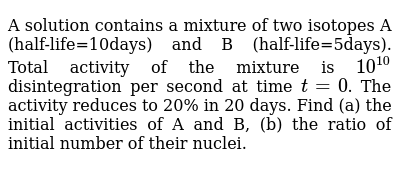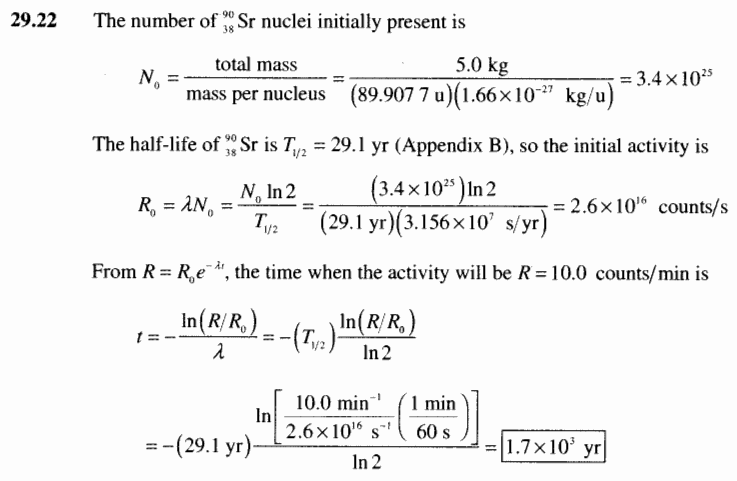How To Find Half Life From Activity
You can find the half life of a radioactive element using the formula.
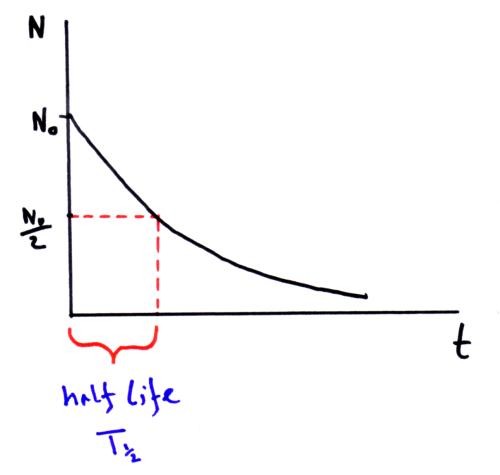
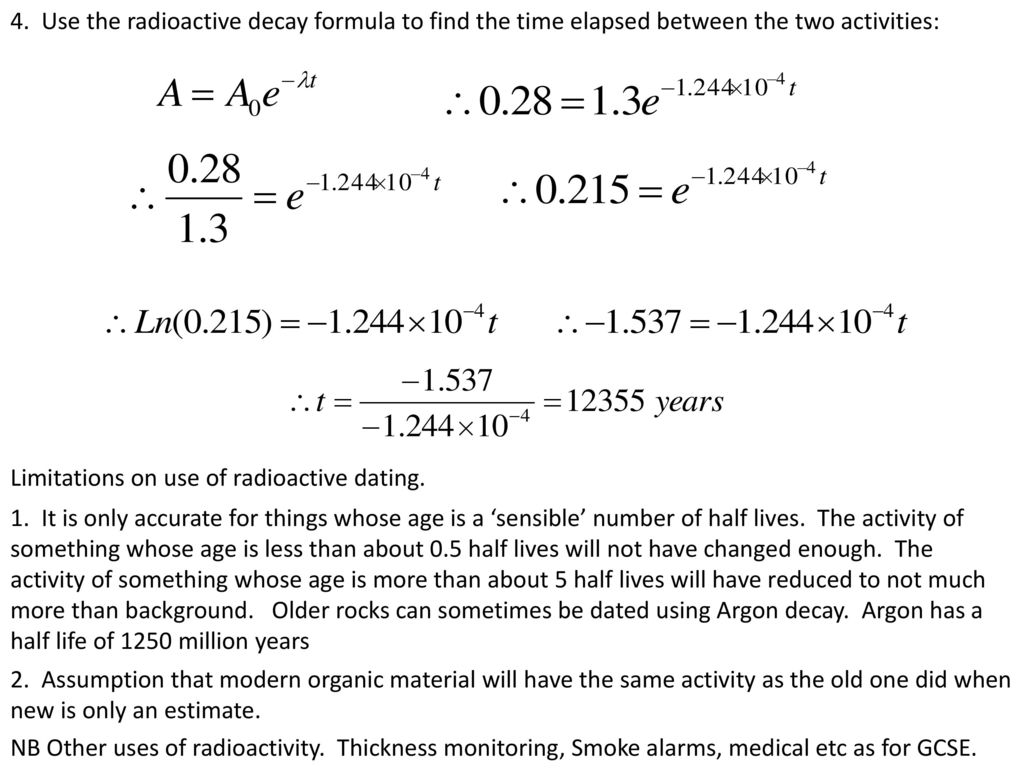
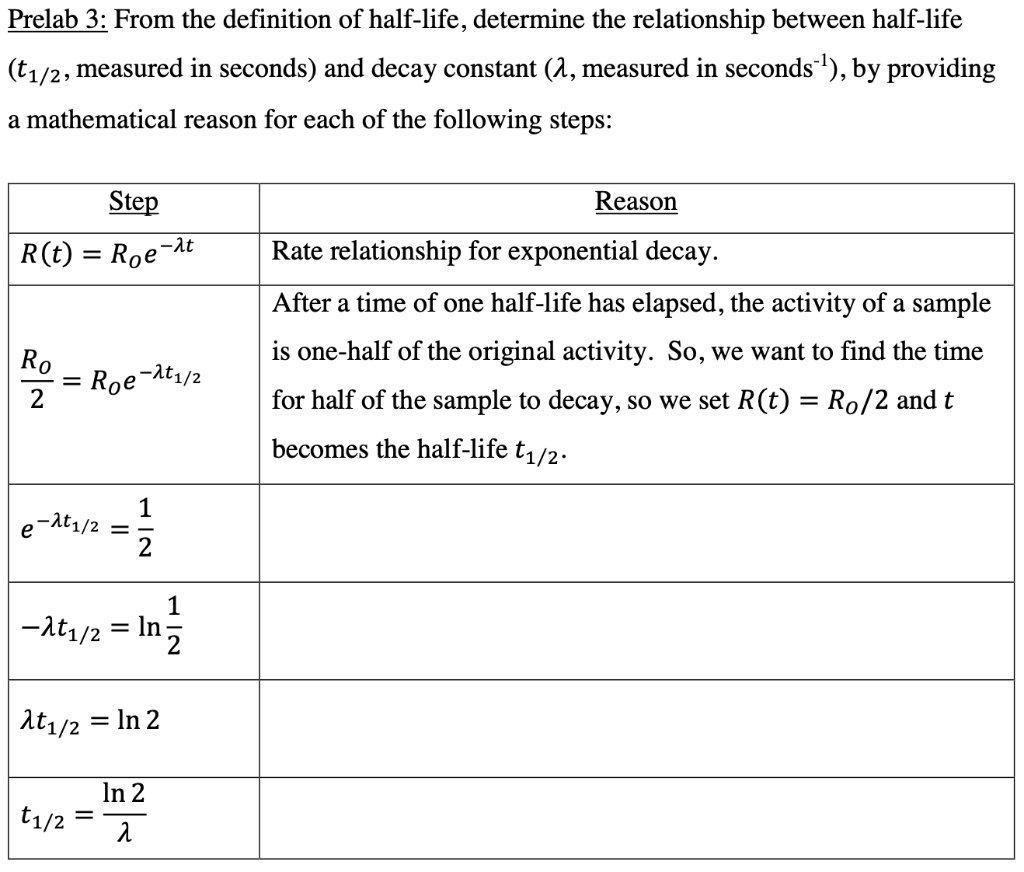
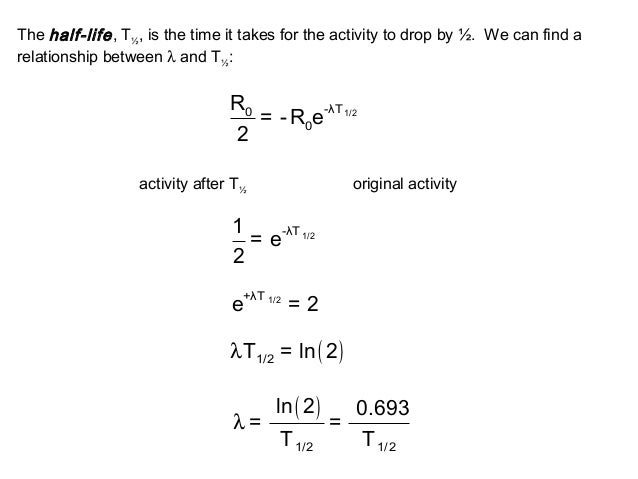
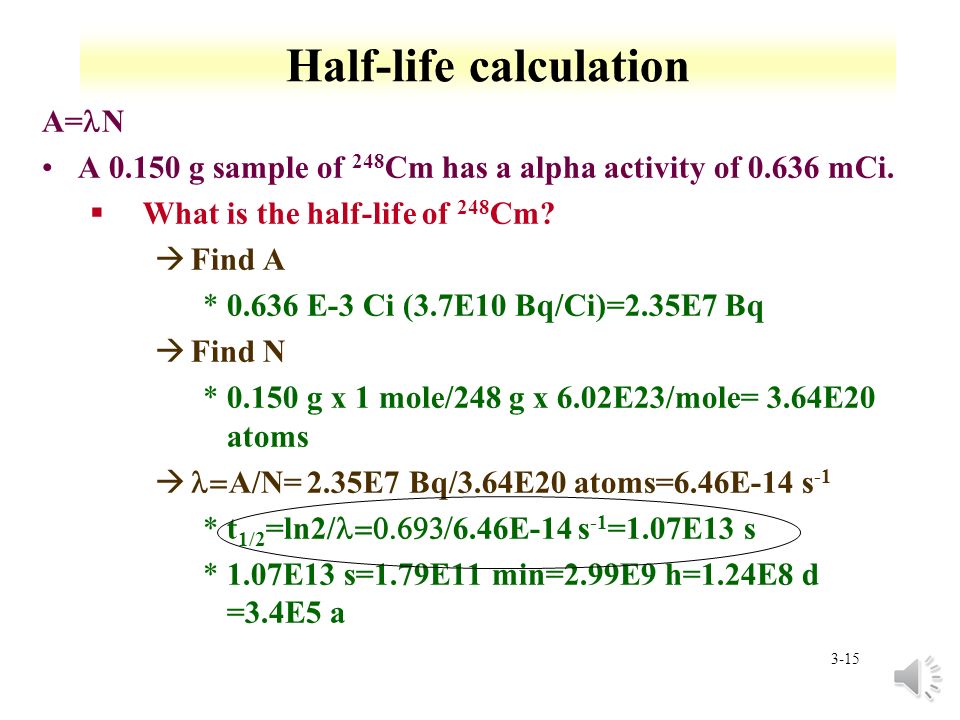
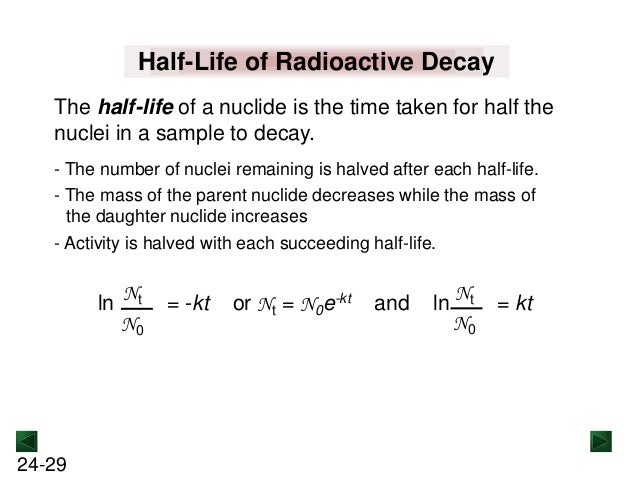
How to find half life from activity. Half life is defined as the time taken for half the original number of radioactive nuclei to decay. N 0 number of undecayed nuclei at t0 t time after t0 in seconds n the number of undecayed nuclei at time t l decay constant s 1 half life. T1 2 ln2 l t 1 2 l n 2 l t 12 is half life. N o is the initial number of radioactive nuclides and.
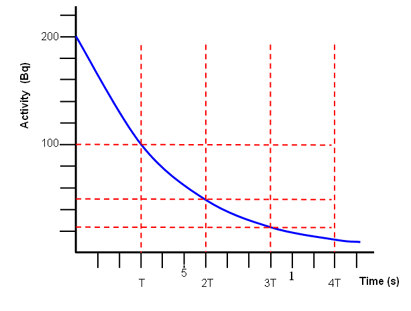
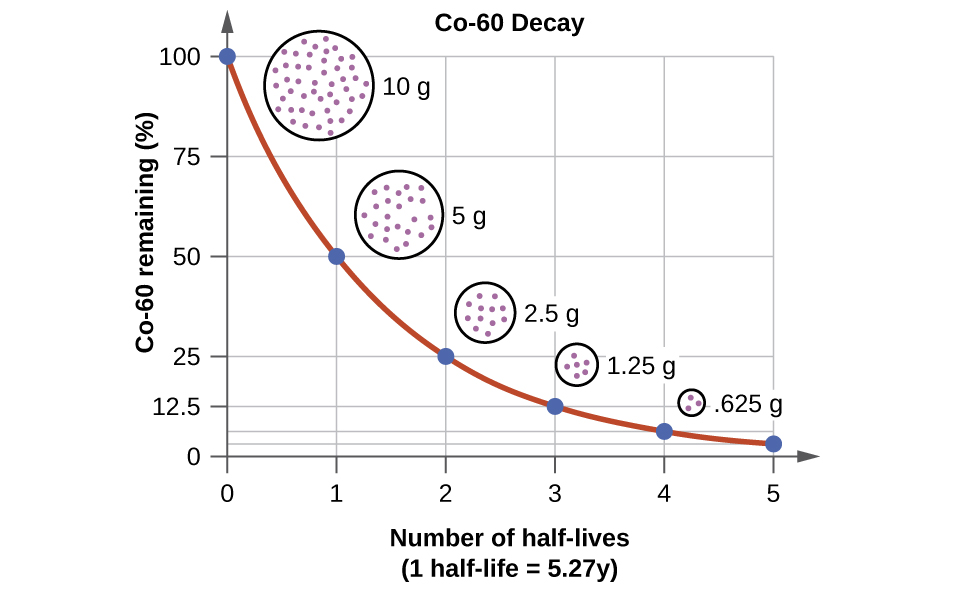
The half life is the time for half the nuclei to decay. A sample contains 13210 24 atoms of indium 112. Scientists also use the half life of carbon 14 to date bones and other organic matter. How to calculate half life.
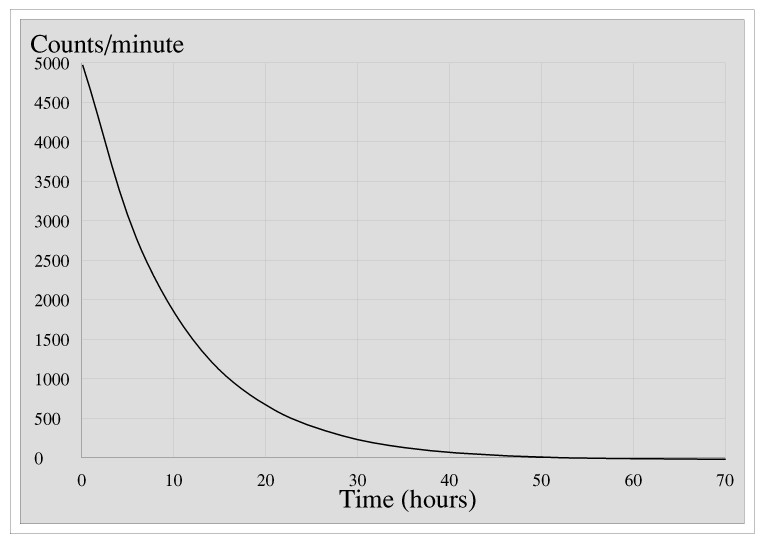
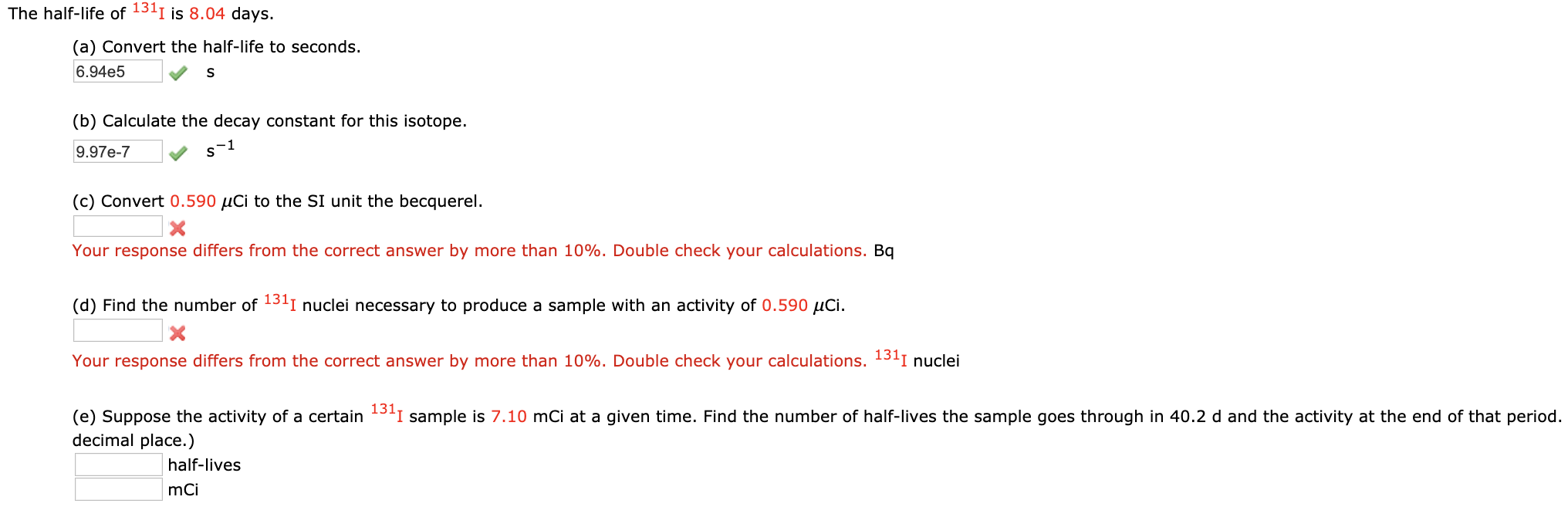
N is the number of nuclides remaining after a time t. A activity in becquerel bq n the number of undecayed nuclei l decay constant s 1 radioactive decay law. The half life of 14c can be found in appendix b and was stated above as 5730 y. You can use the half life equation to calculate how long radioactive waste will remain dangerous.
A since b using atoms. N n oelt n n o e l t where. Indium 112 has a half life of 144 minutes. B find out how many atoms of indium 112 would be left in the sample after 1 hour.
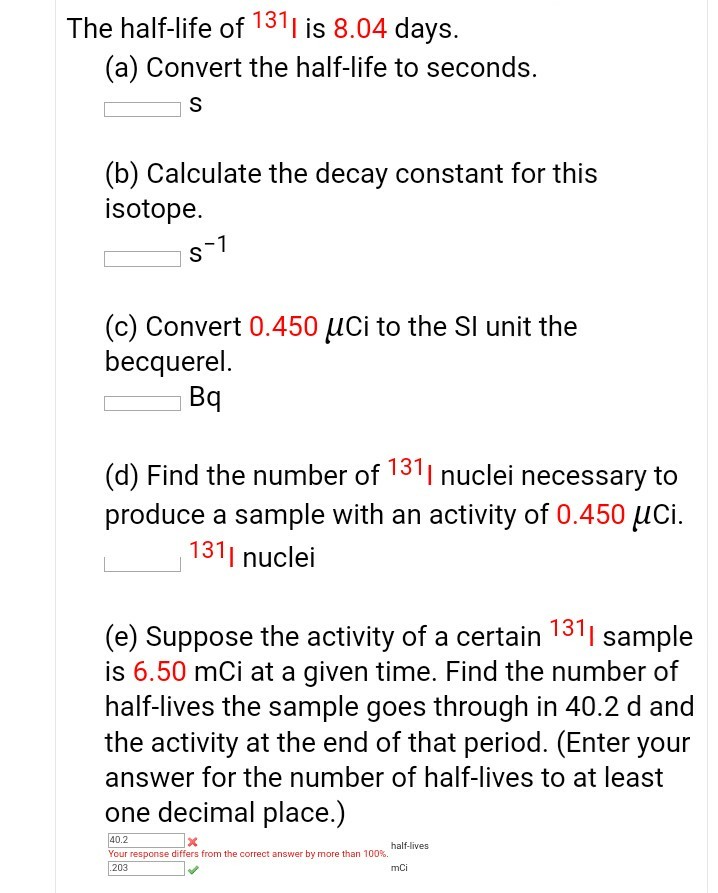
To find n we first find the number of 12c nuclei in 100 kg of carbon using the concept of a mole. This equation is used in the calculator when solving for half life time. To find n we first find the number of 12c nuclei in 100 kg of carbon using the concept of a mole. To find the activity r using the equation r 0693n t1 2 we must know n and t1 2.
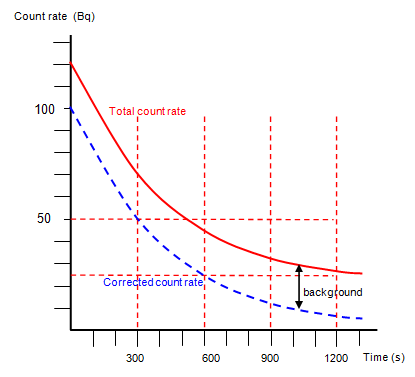
The half life of a sample of radioactive material is the time it takes for half of the sample to decay. A find the decay constant. To find the activity r using the equation r 0693n t1 2 we must know n and t1 2. Where t12 is the half life of the particle t is the elapsed time n0 is the quantity in the beginning and nt is the quantity at time t.
The half life of 14c can be found in appendix b and was stated above as 5730 y. One quick way to do this would be to figure out how many half lives we have in the time given. 6 days2 days 3 half lives 1002 50 1 half life 502 25 2 half lives 252 125 3 half lives so 125g of the isotope would remain after 6 days.



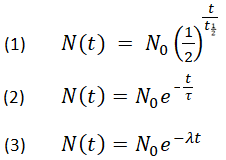
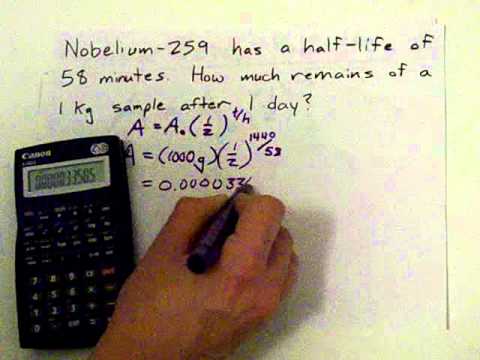



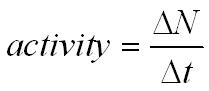

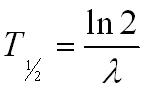
/ThoughtCo_List_Of_Radioactive_Elements_608644_V12-e91d220318ed4143a7ff5a9407af6555.png)