How To Do Titration Problems
When chemists need to find the concentration of a substance dissolved in a solution they often use a technique called titration.
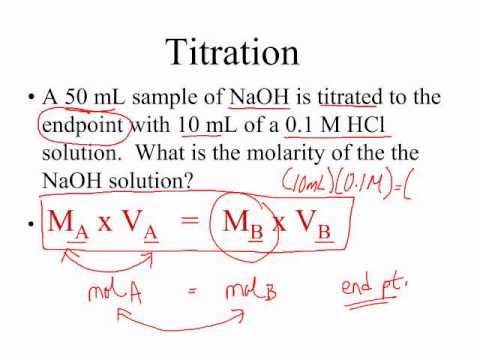
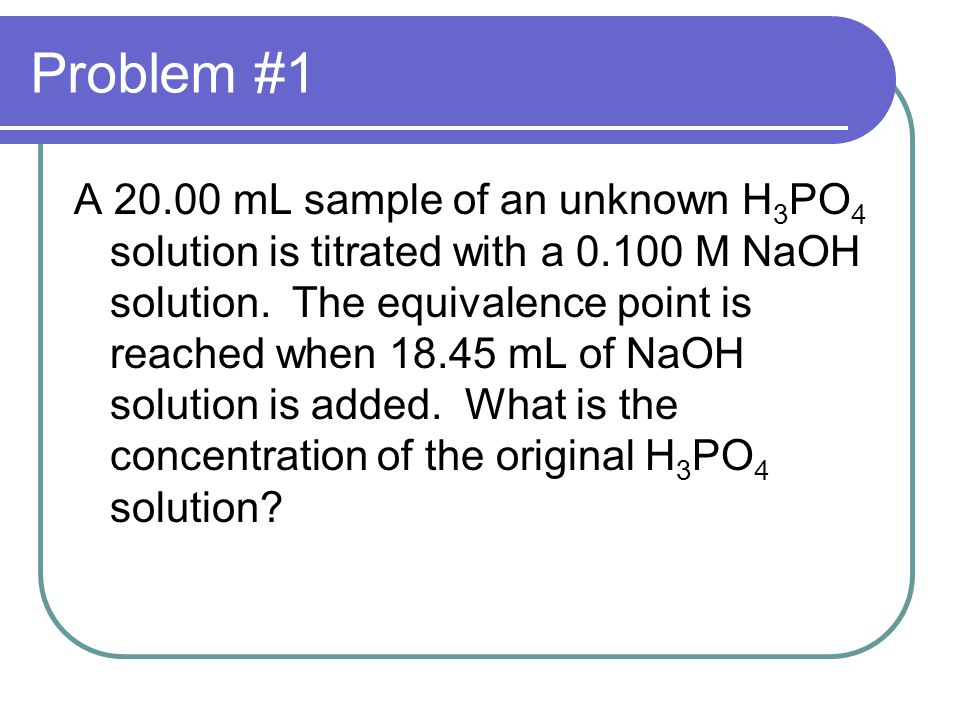
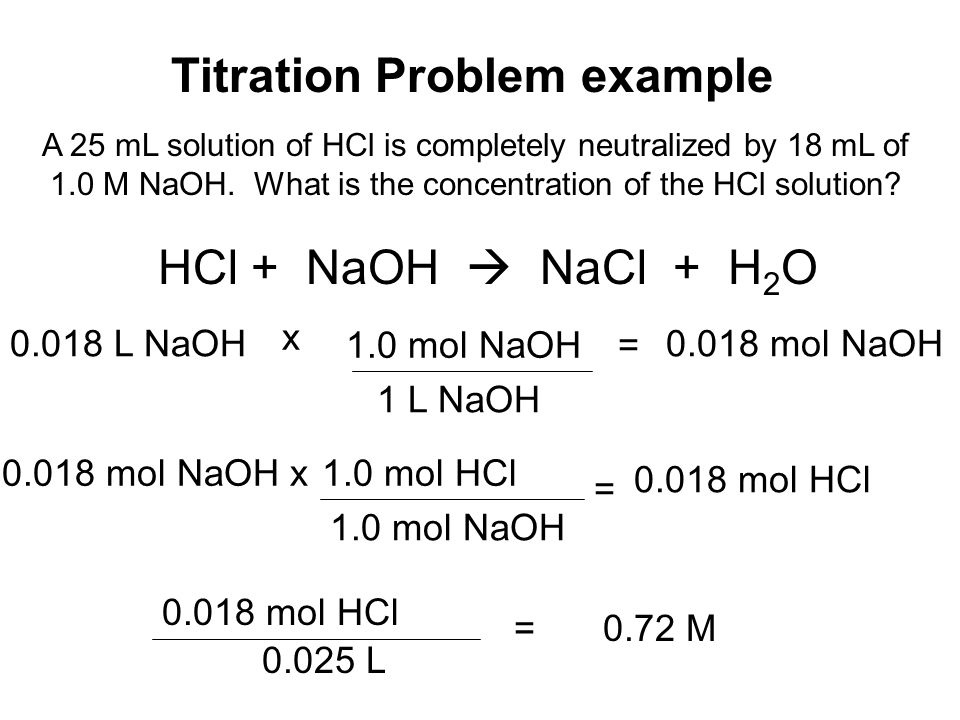
How to do titration problems. Moll 1 are the units for concentration. Titration problems with acids and bases are common assignments on homework and tests in chemistry class. Therefore oh 05 m. Number of moles molarity x volume.
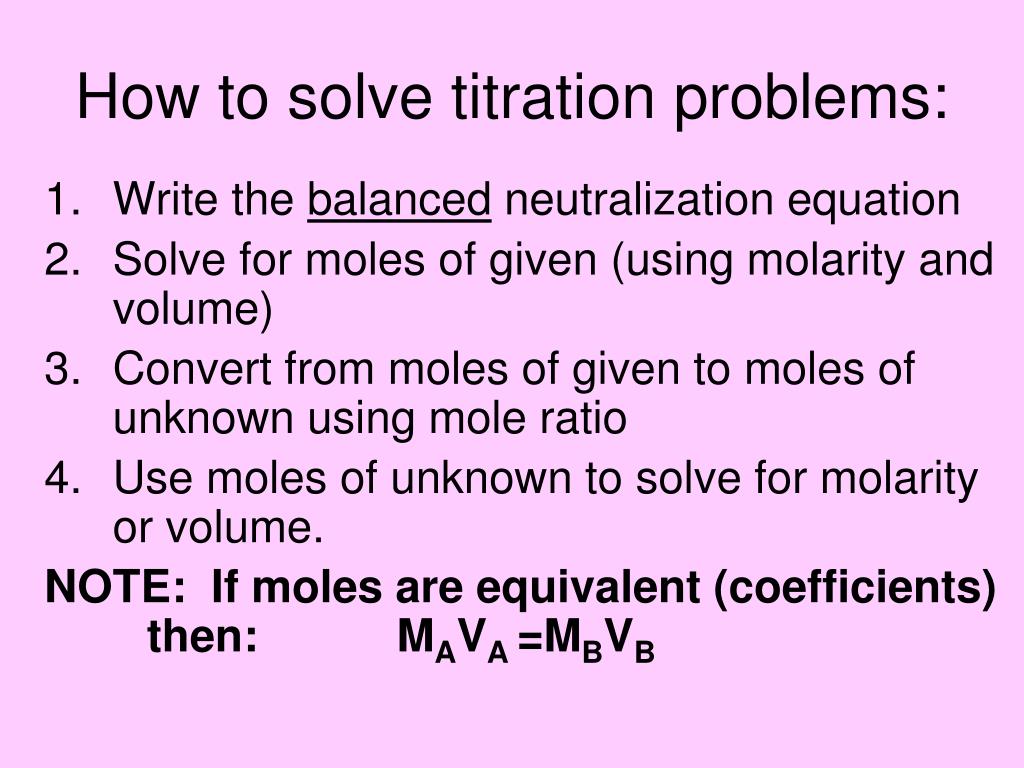
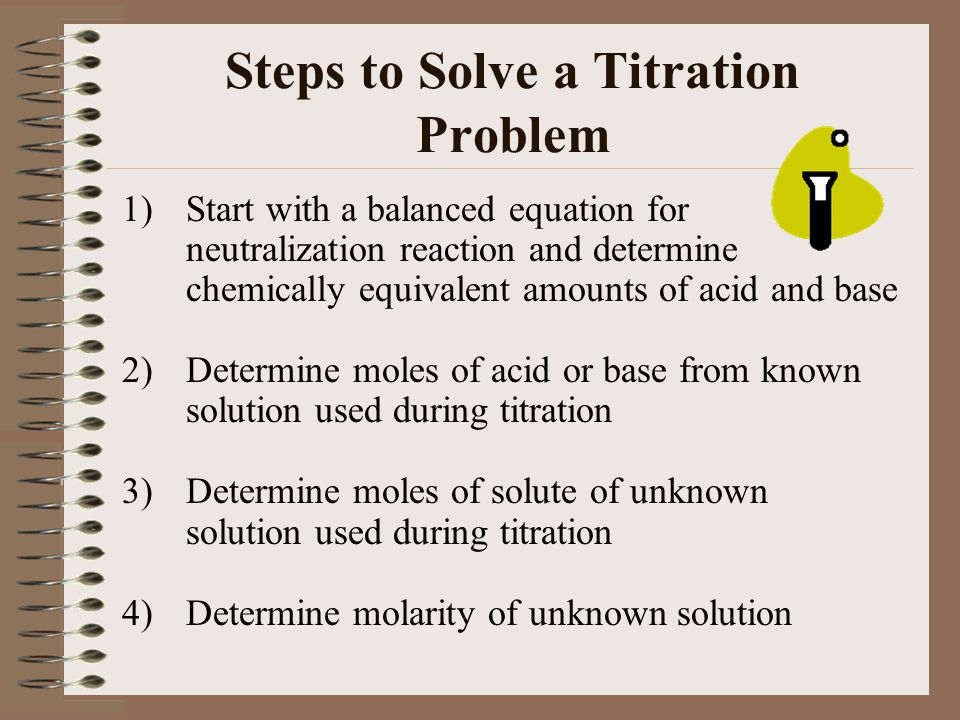
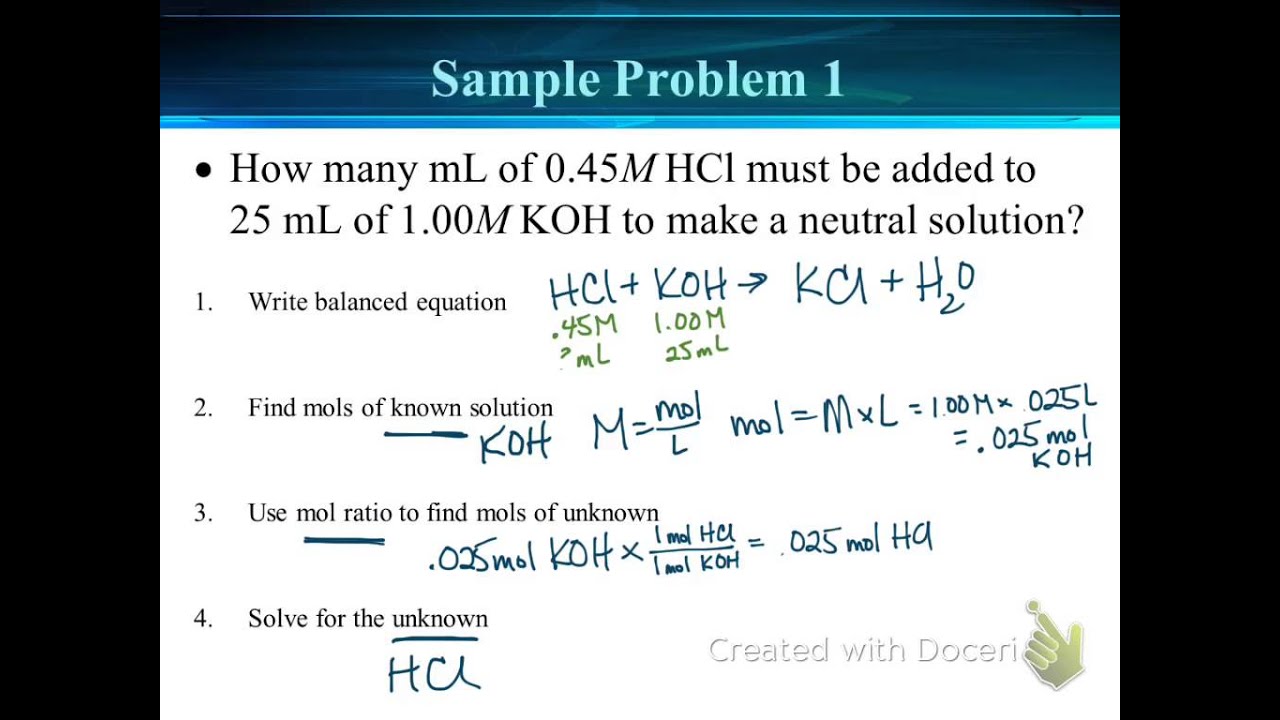
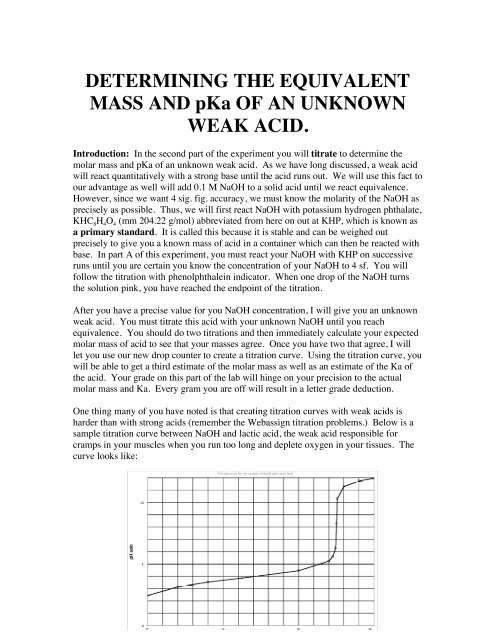
Solve for equilibrium concentrations using ice tables or henderson hasselbalch approximation. We use the relationship moles massmolar mass and molarity concentration moles of stuffvolume of solution now when we use molarity we can preserve the dimensions. The molecular weight of the unknown is 1891 gmol. Calculate the number of moles of base to know the number of moles of the unknown because it is a monoprotic acid.
Determine the number of moles of oh. Use stoichiometry of the neutralization to determine the amounts of acid and conjugate base present in solution step 2. Titration problem step by step solution. Once you know the number of moles of the unknown divide the mass of the unknown by the number of moles to obtain the solution.
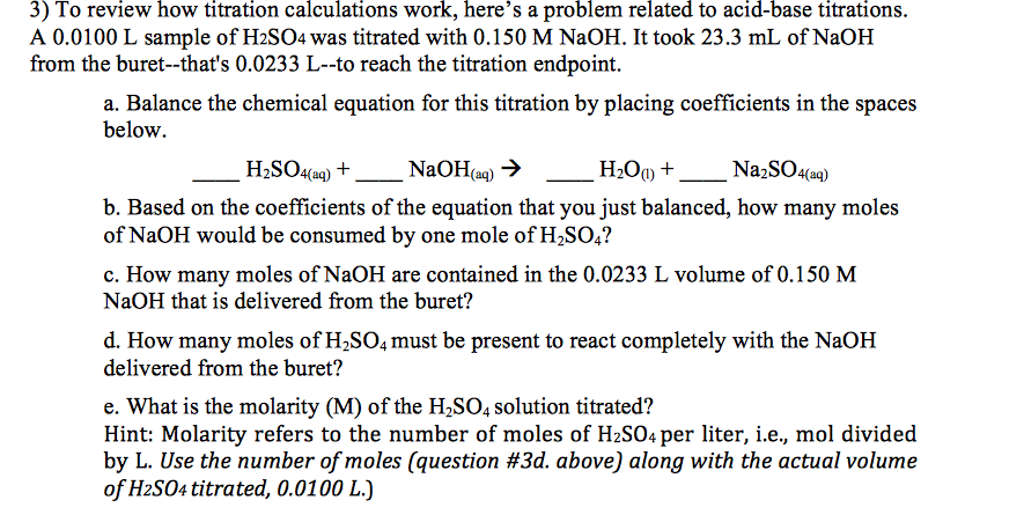
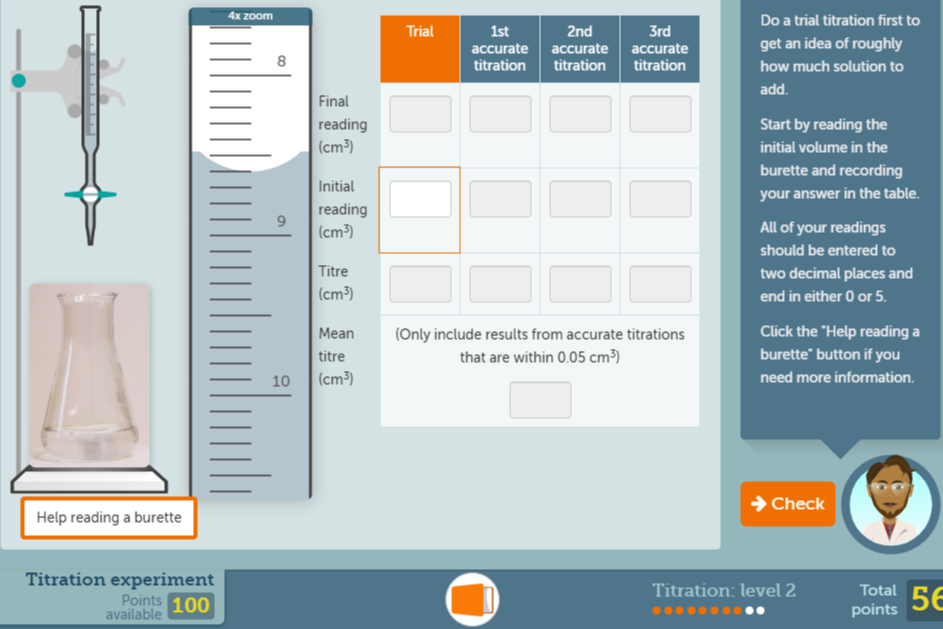
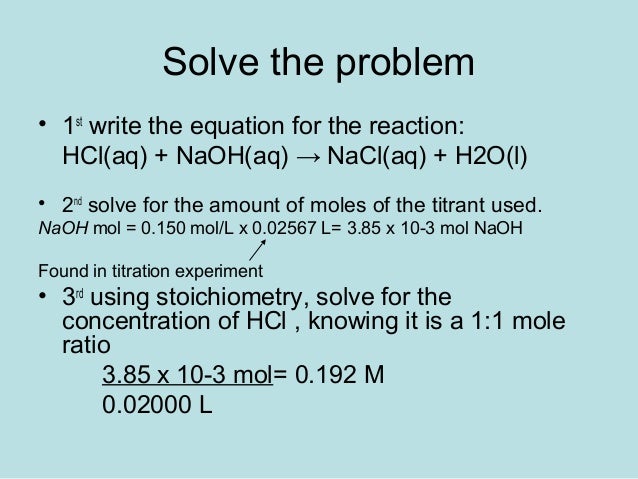
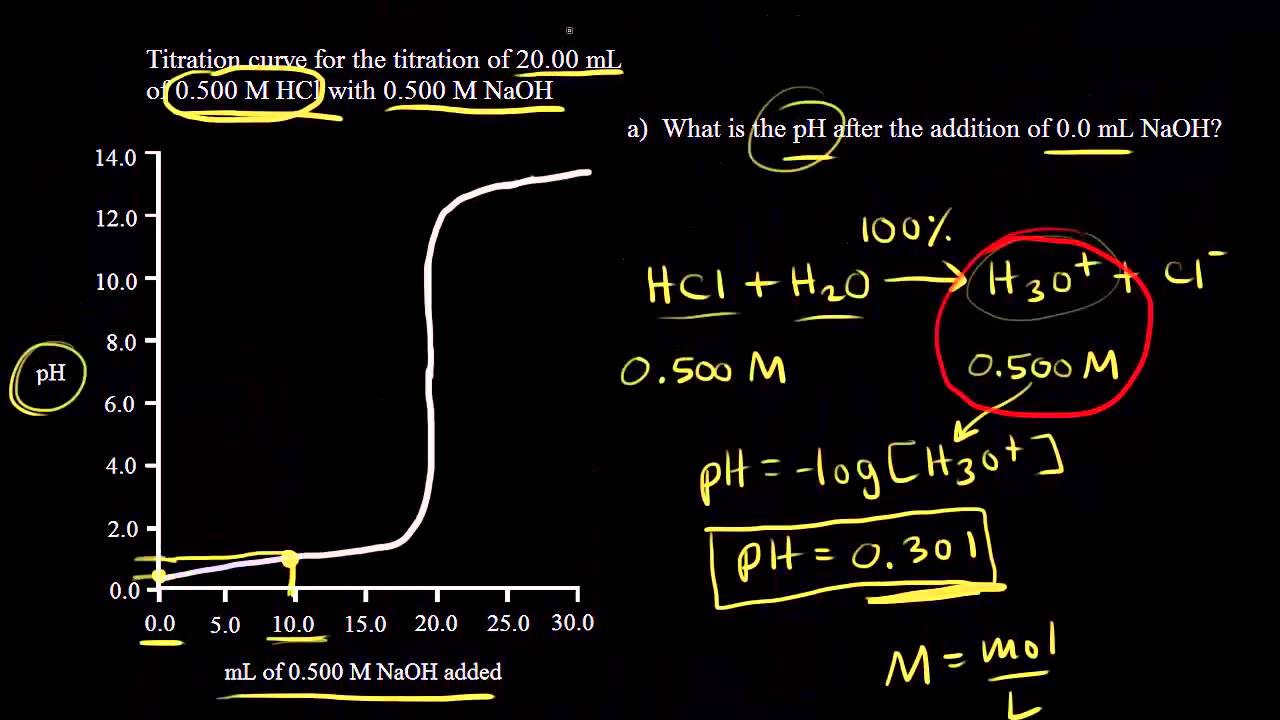
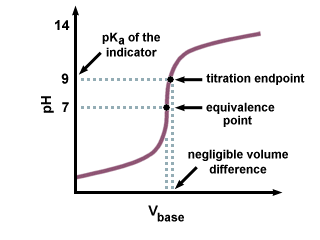
In titration one solution solution 1 is added to another solution solution 2 until a chemical reaction between the components in the solutions has run to completion. With a titration a measured quantity of titrant is added to a known mass of known molar quantity. Titration stoichiometry problems do not get much trickier than this. Solution 1 is called the titrant and we say that it is used to titrate solution 2.
Number of moles oh 05. Determine oh every mole of naoh will have one mole of oh. How to calculate the unknown concentration when you dont have a 11 molar ratio of h to oh. By adding a chemical that reacts with the solute until all of the solute has been neutralized the chemist can determine how much was originally present and hence the concentration of the solution.
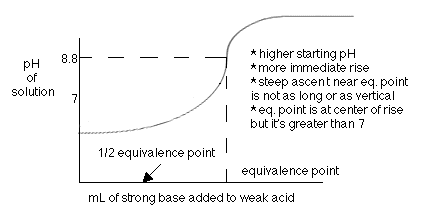
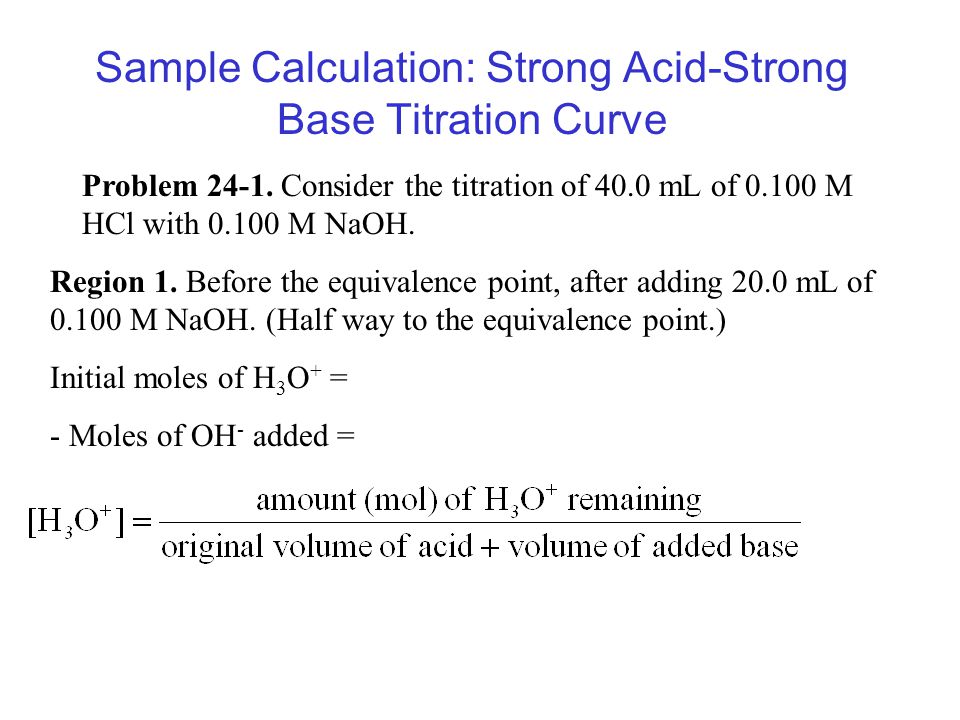
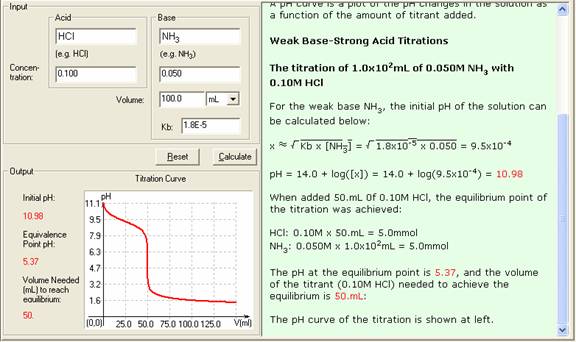
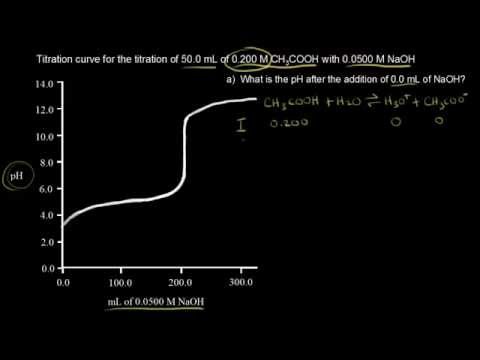
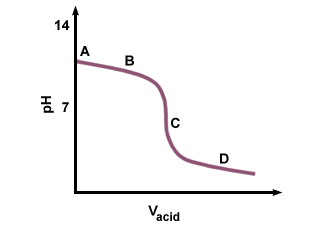
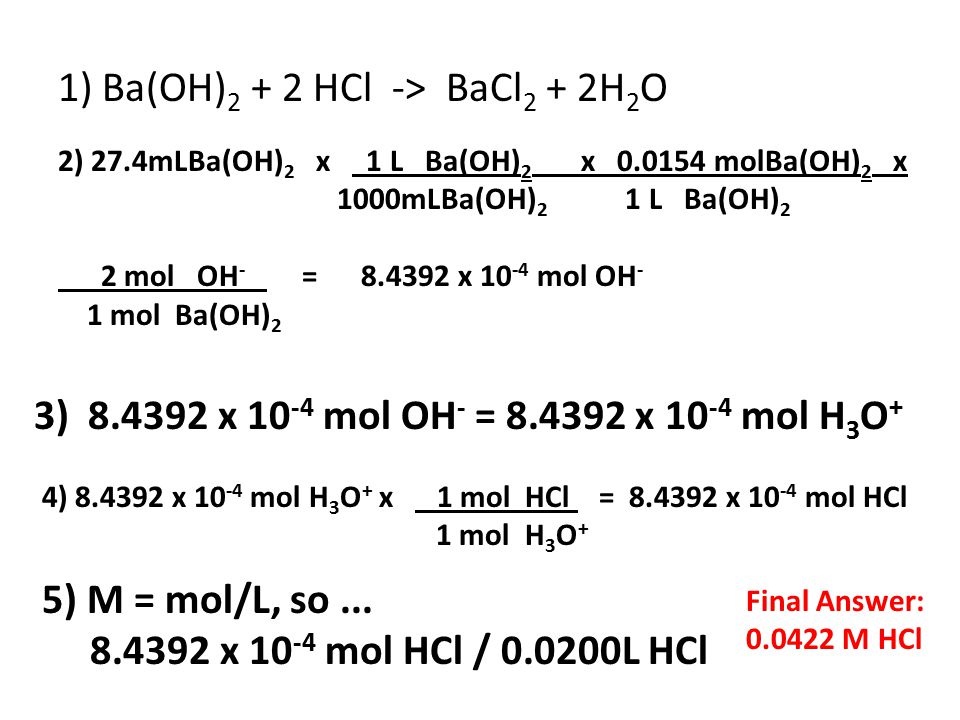
Example of titrating strong acid hydrochloric acid with strong base barium hydroxide. Calculating the ph during the titration of a weak acid or a weak base step 1.
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gctvvjq2l Mlfrtapckqnvwezss4vmvnj5 0uvgaryglwav2yhzs Usqp Cau

What Is Purity Definition How To Do Percent Purity Calculations Gcse Chemistry Calculations 14 Igcse Ks4 Science A Level Gce As A2 Practice O Level Questions Exercises































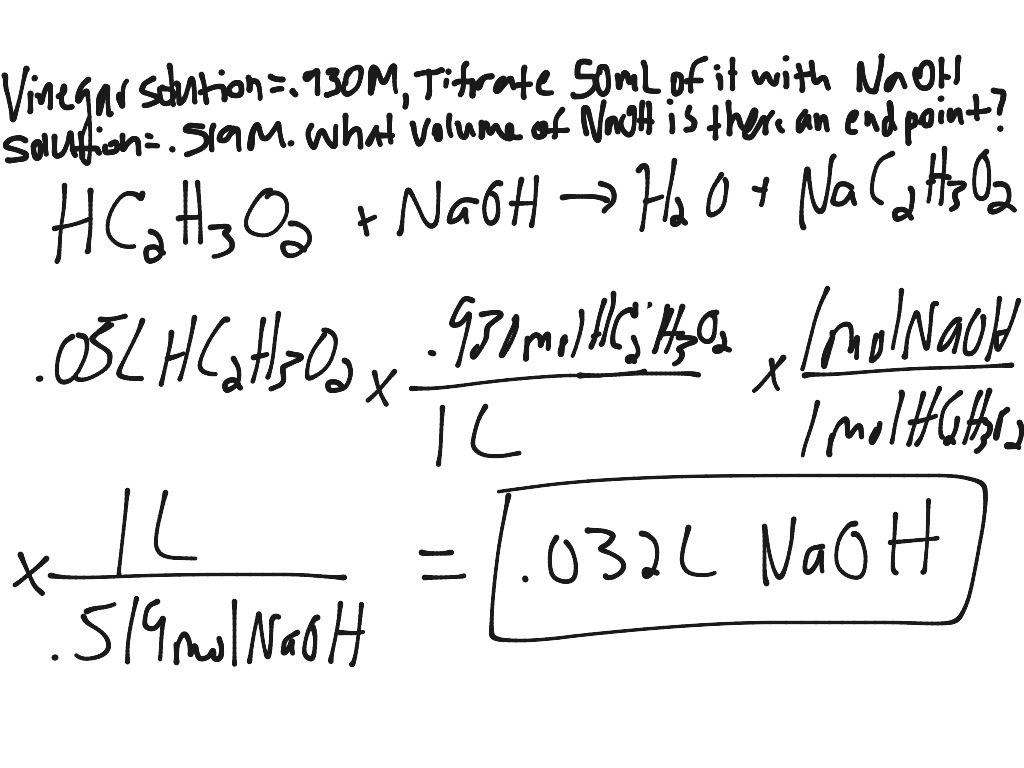


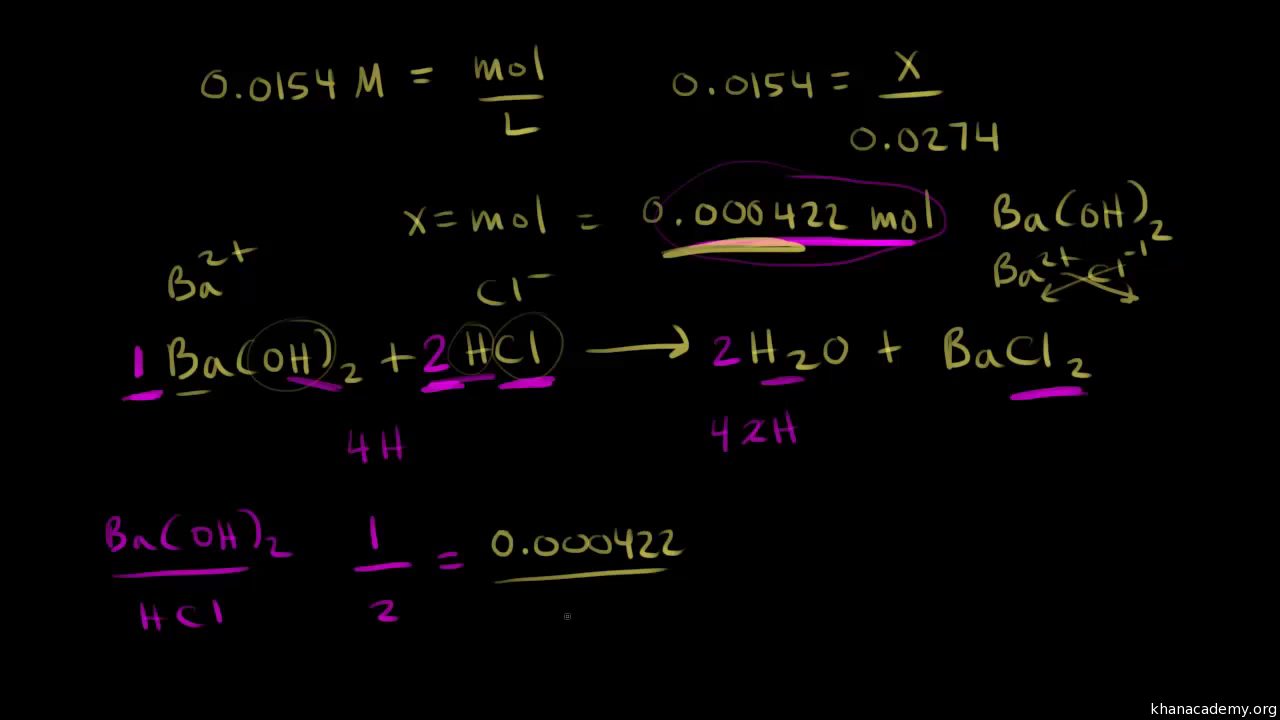


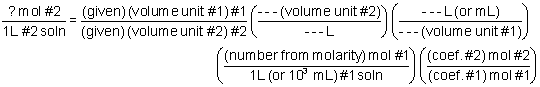



:max_bytes(150000):strip_icc()/how-to-calculate-normality-609580final2-0d5efa5a961f4fa0a7efc780921faee1.png)









.PNG)
/chemistry-research-172591498-58bc66a15f9b58af5c6c24e1.jpg)