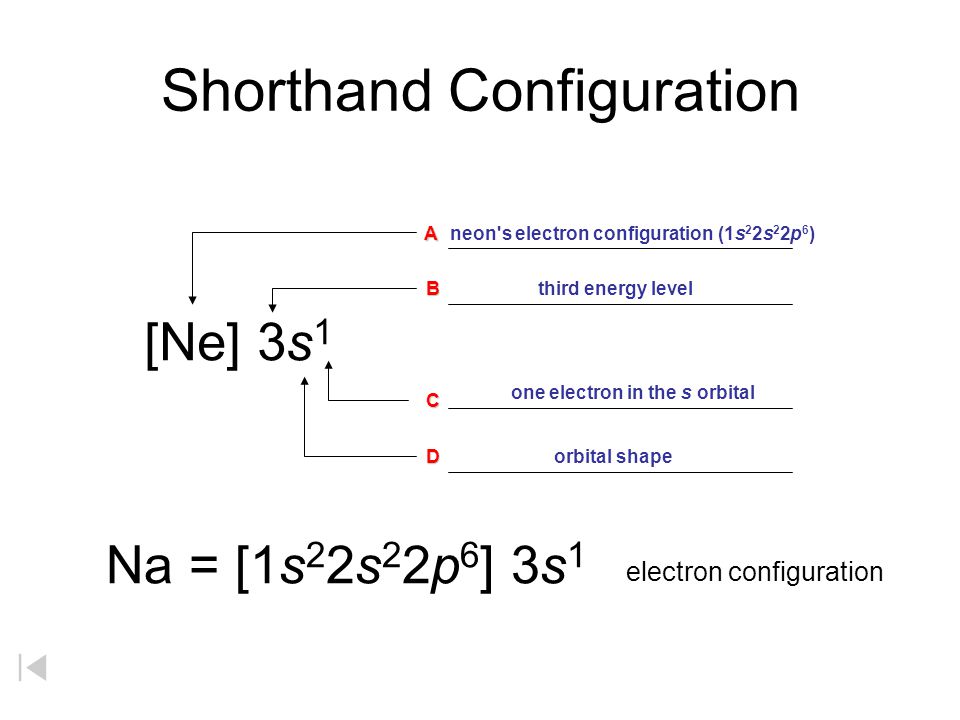
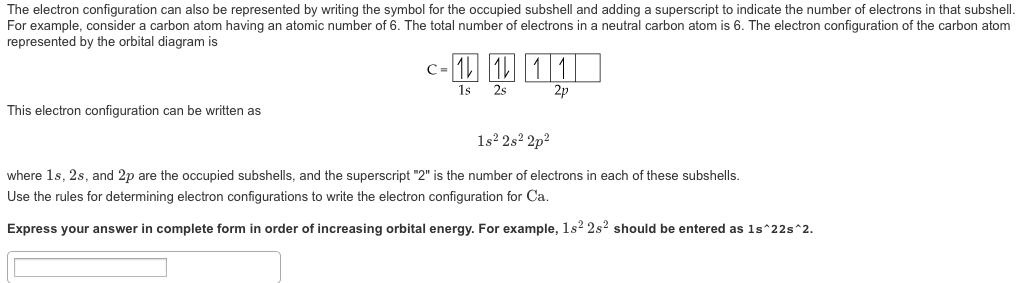
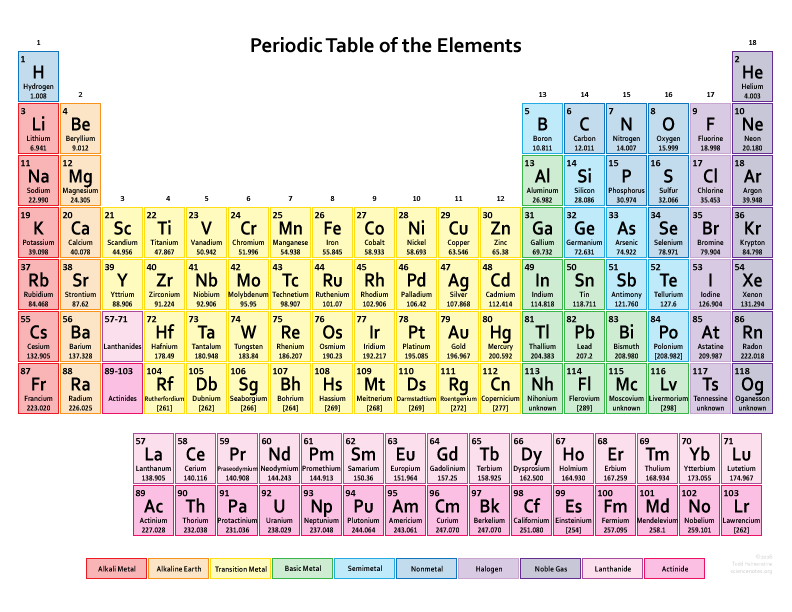
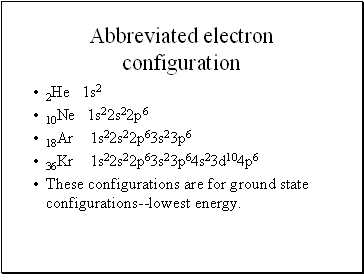
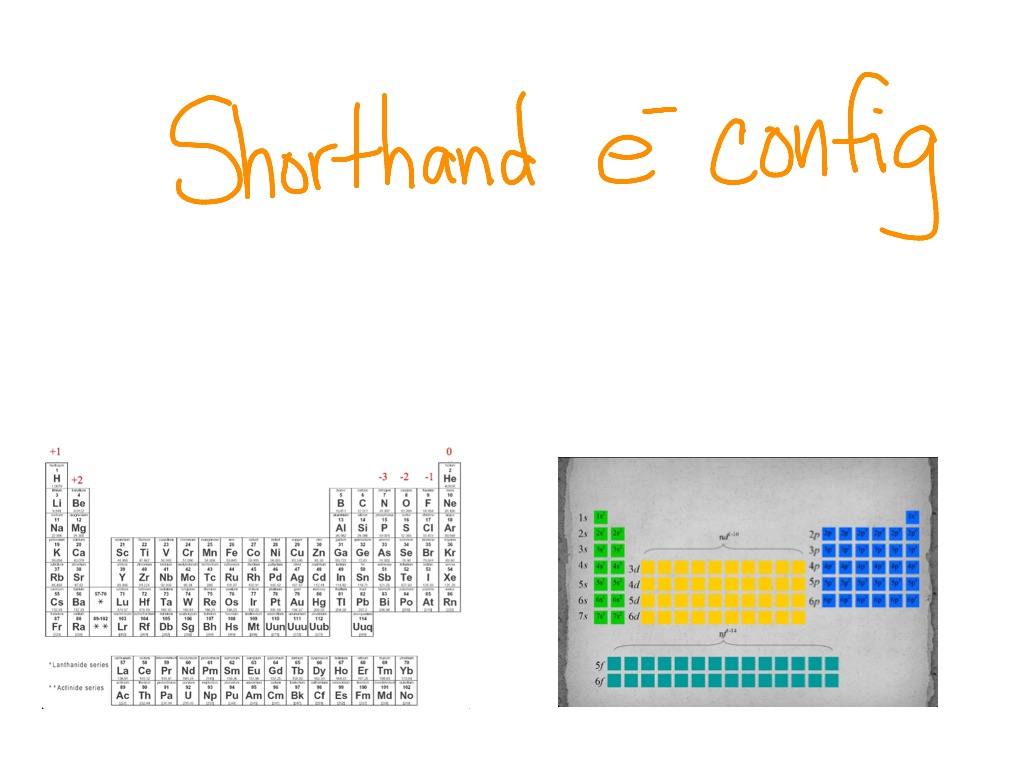
How To Do Electron Configuration Shorthand
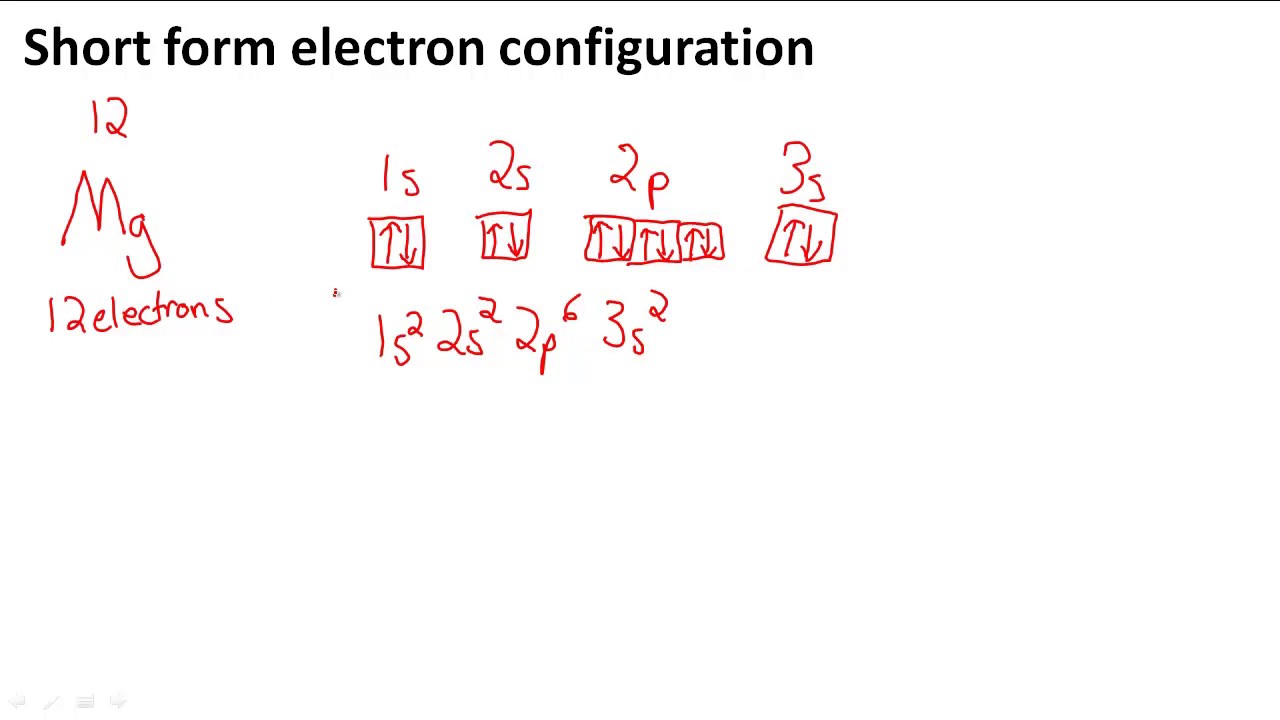
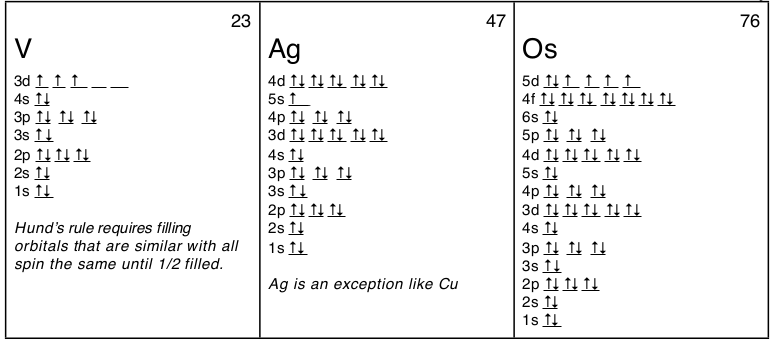
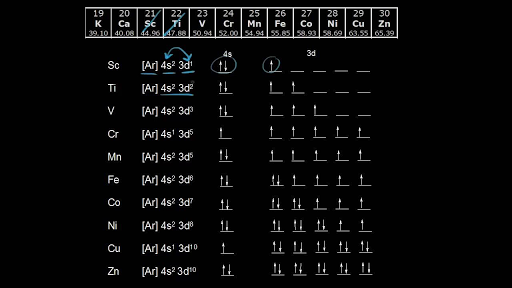
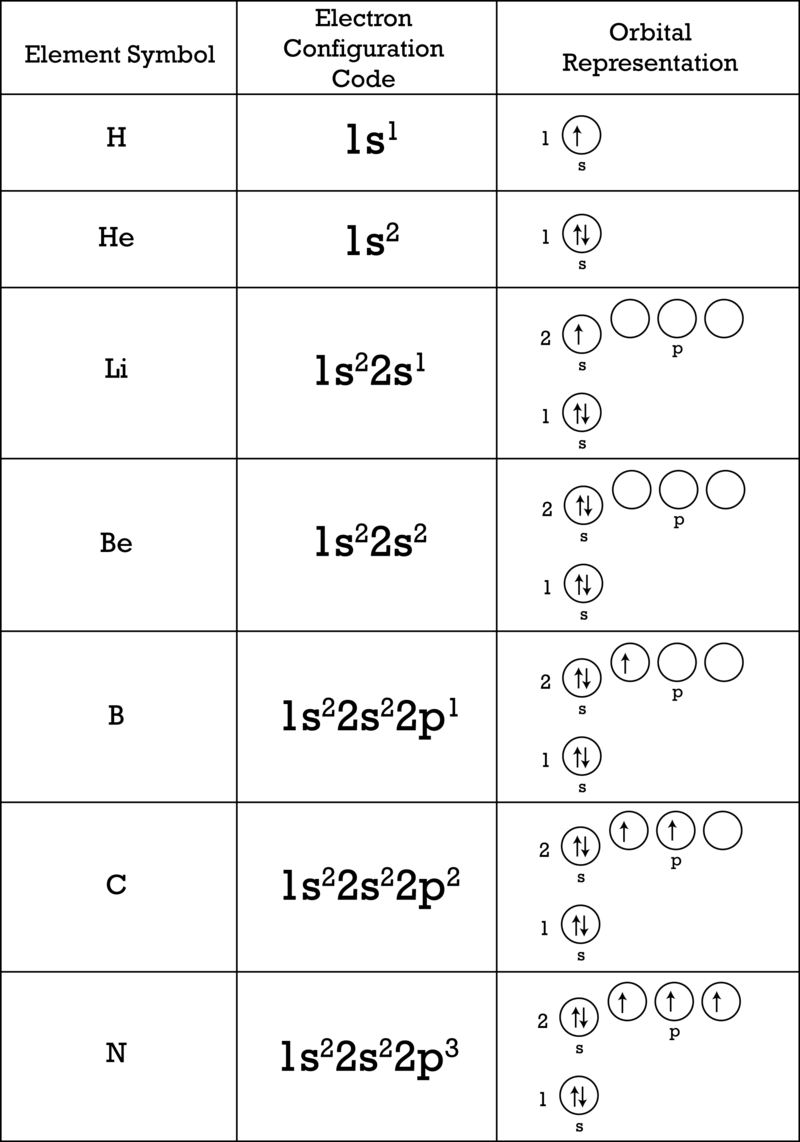
In writing the electron configuration for iron the first two electrons will go in the 1s orbital.
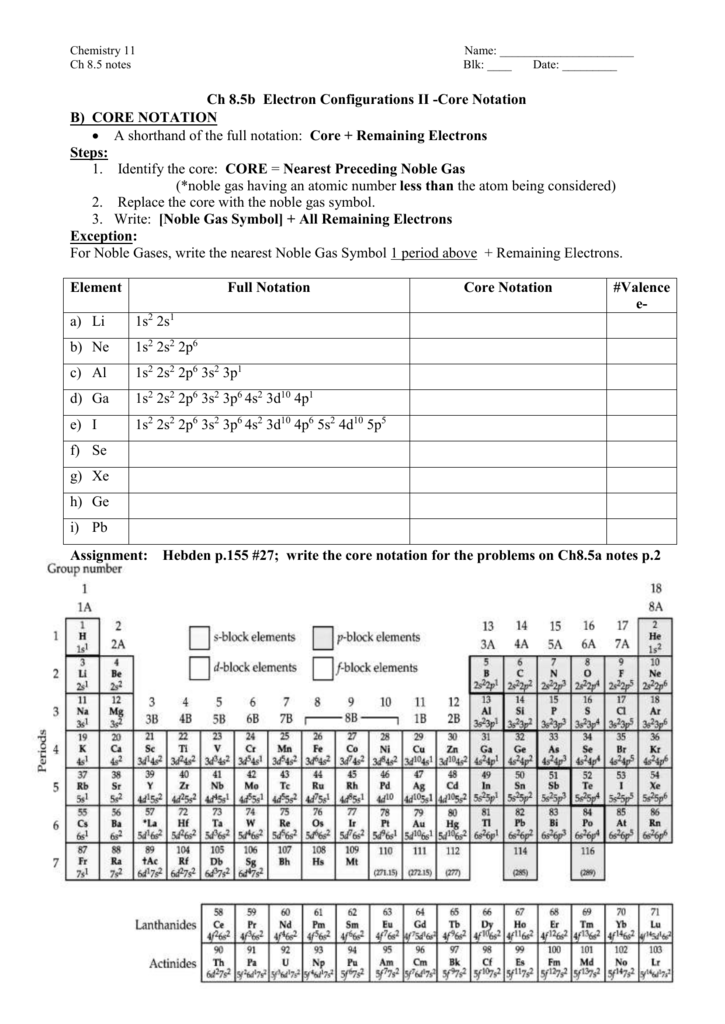
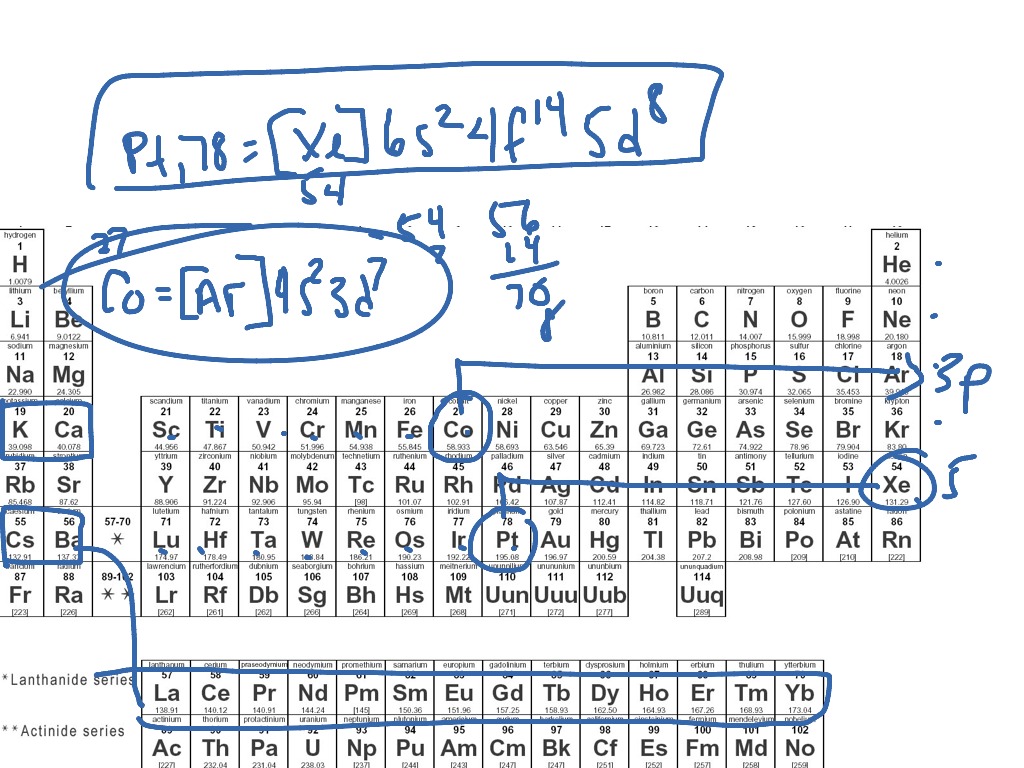
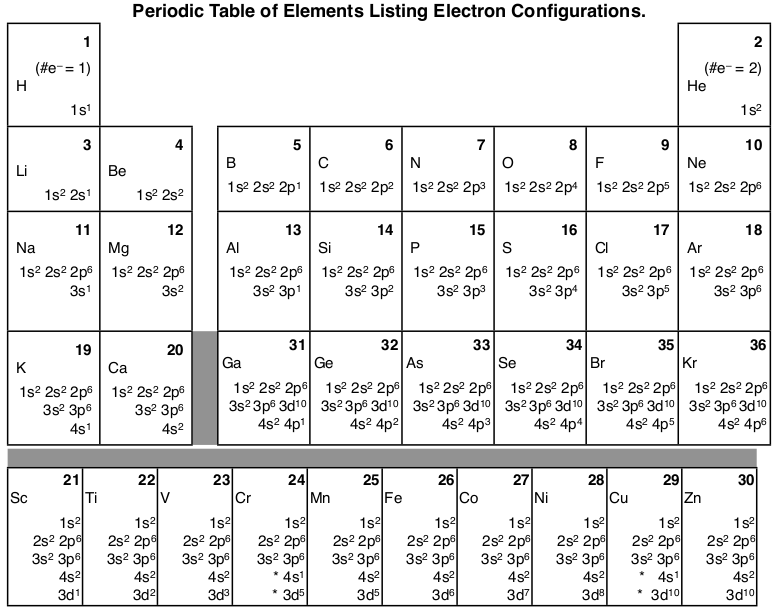
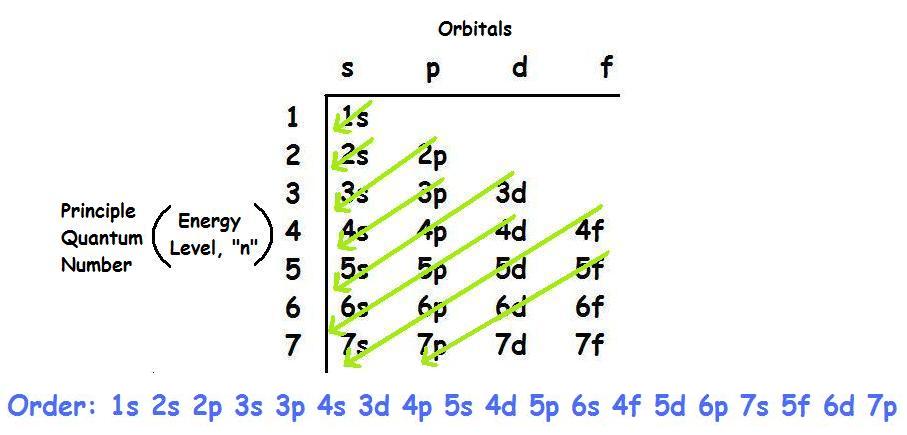
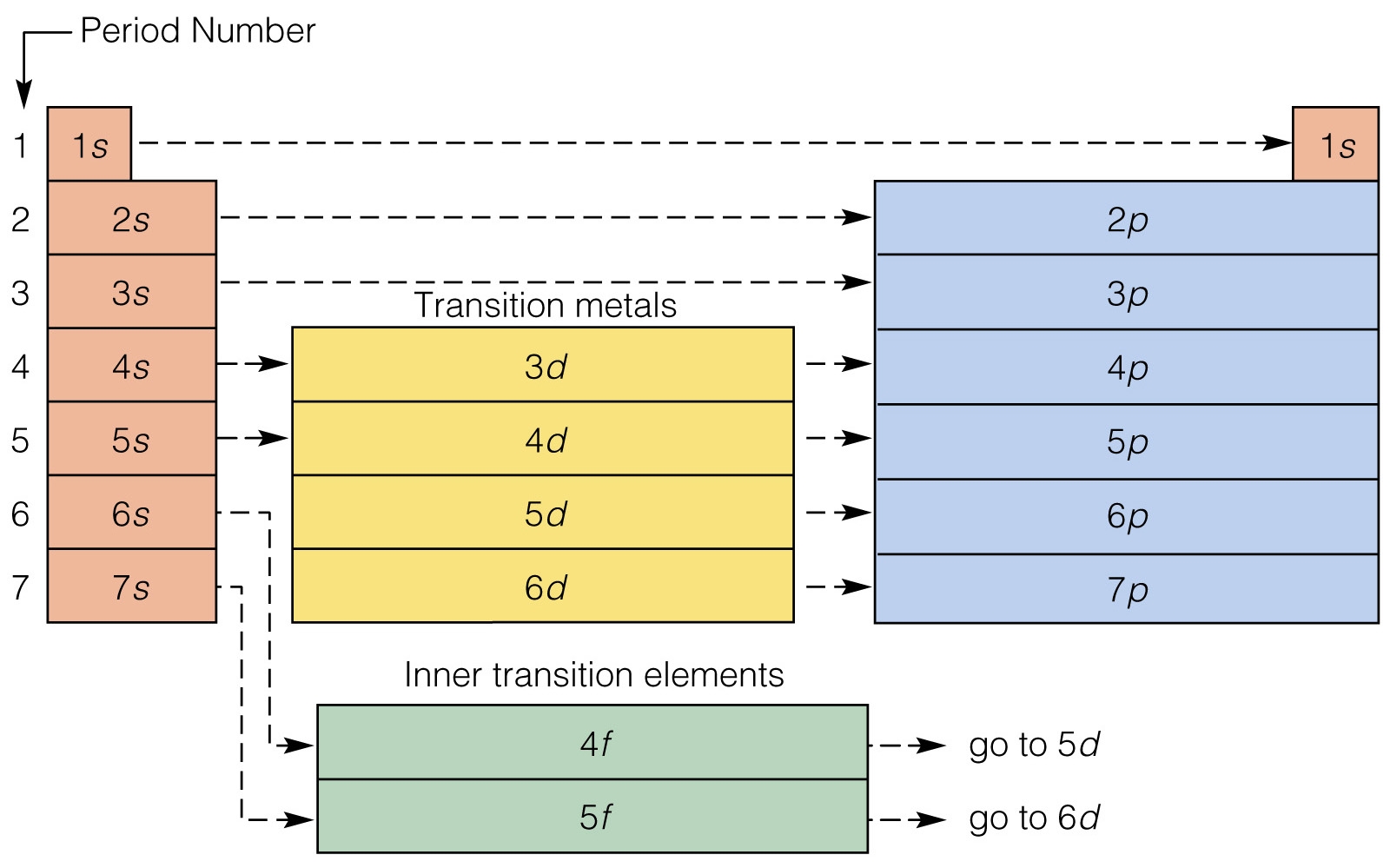
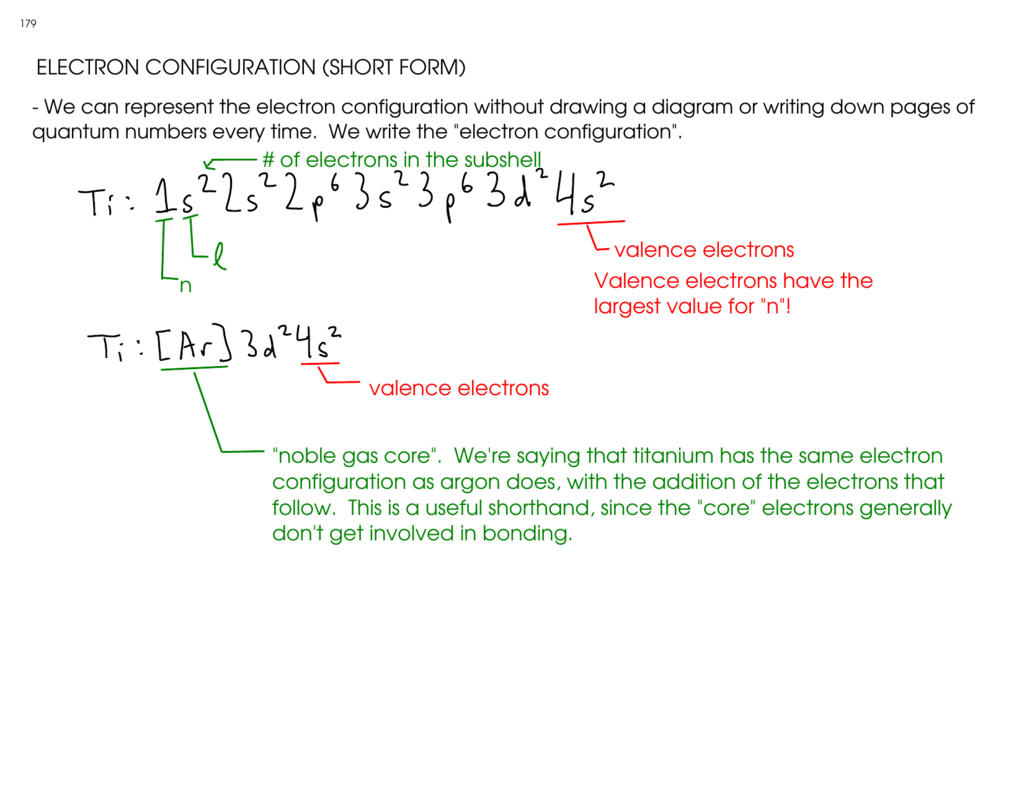
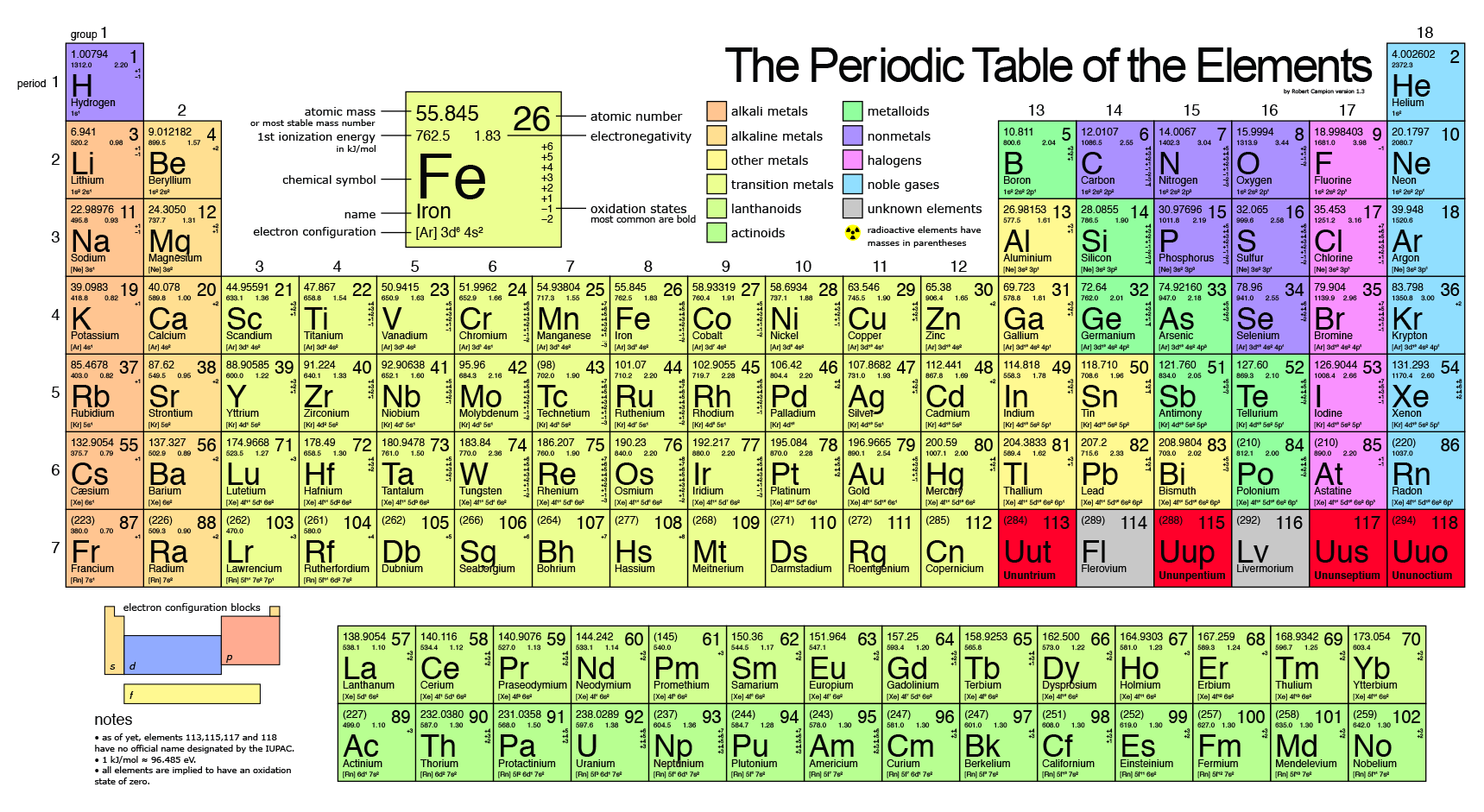
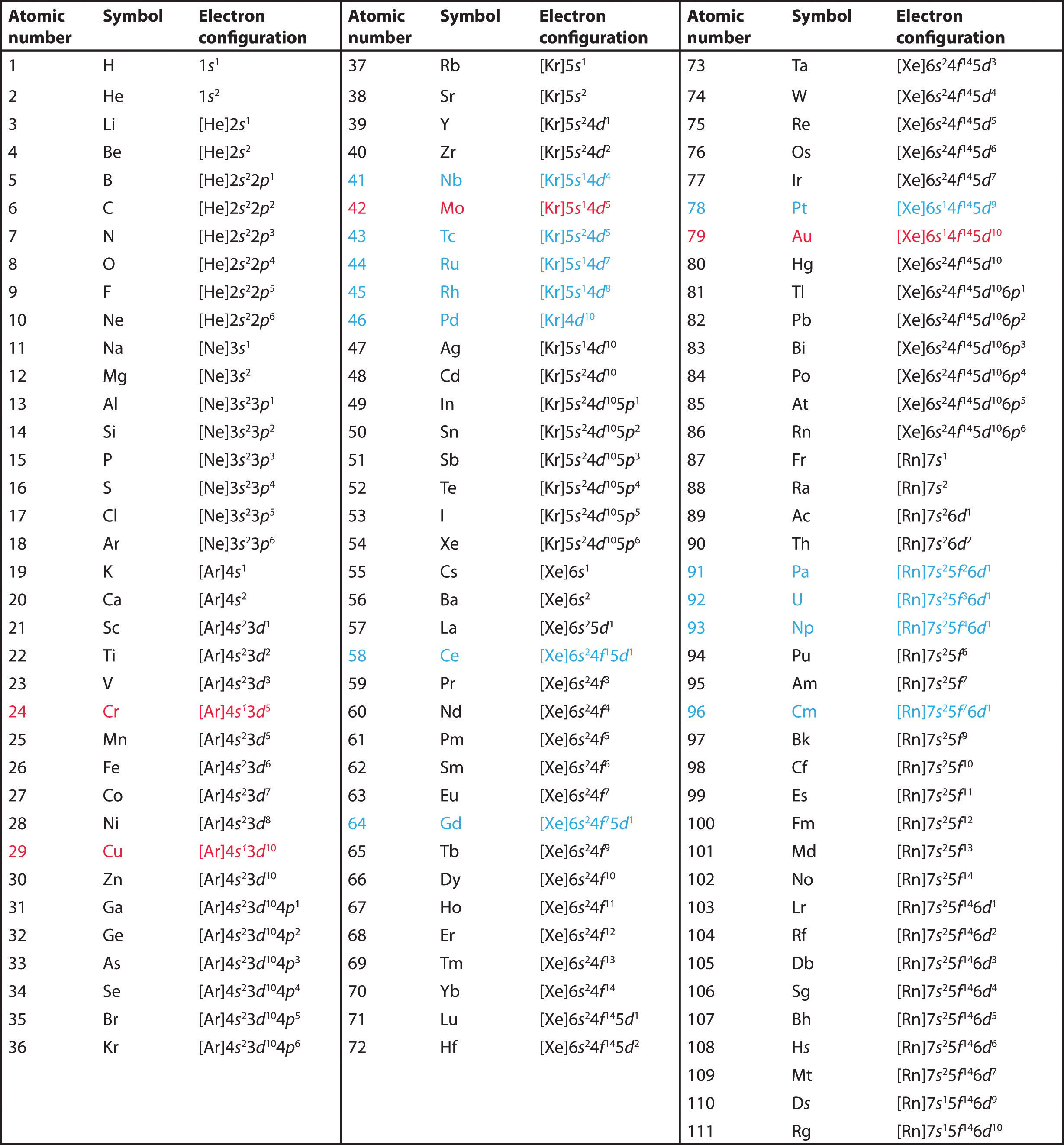
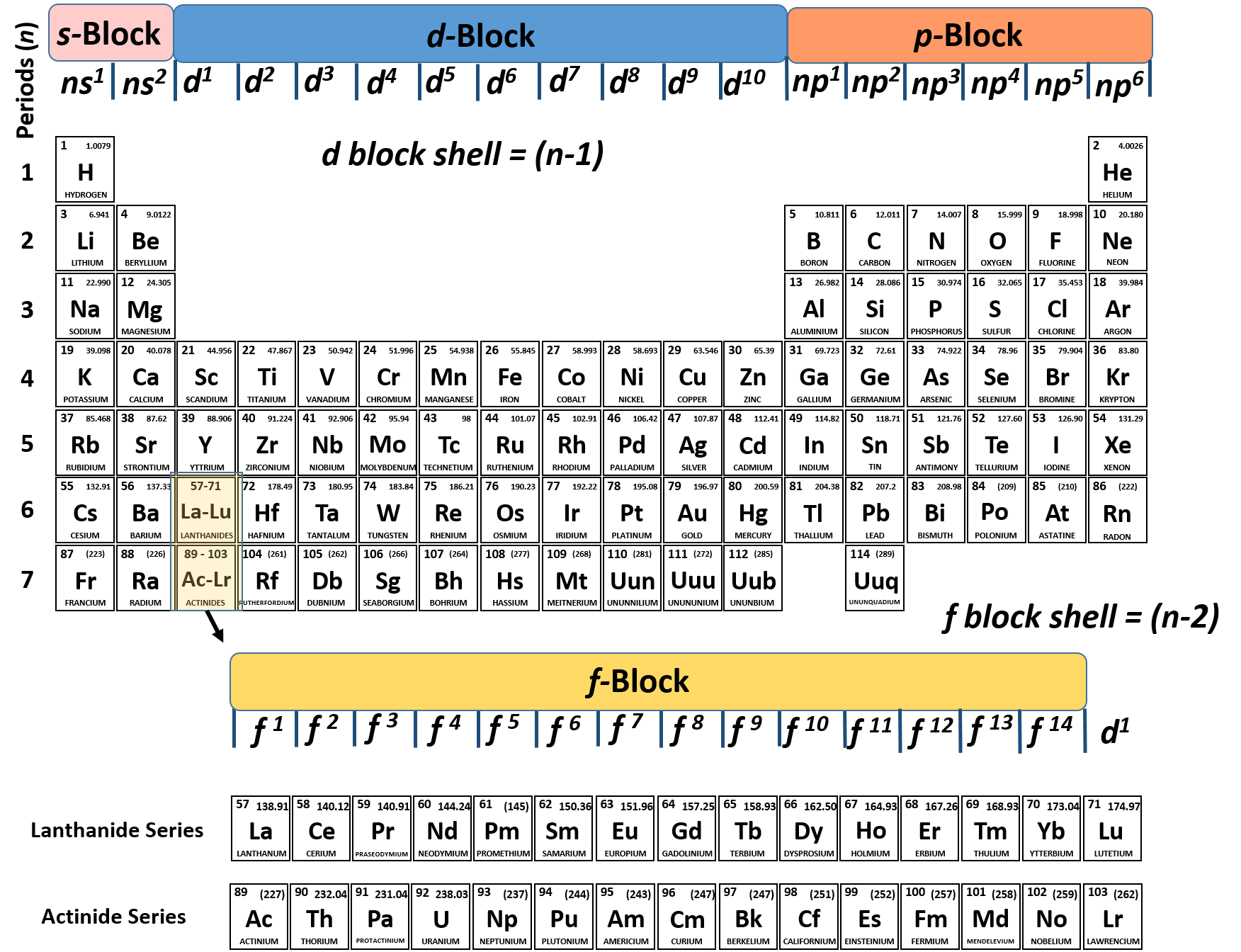
How to do electron configuration shorthand. Electron configurations have the format. Shorthand notations make use of the fact that the noble gases have full outer electron shells and some sources call it noble gas notation for this reason. For example if you need to write electron configuration of erbium 68 cross out elements 69 through 120. Go back to the last noble gas that was passed atomic number.
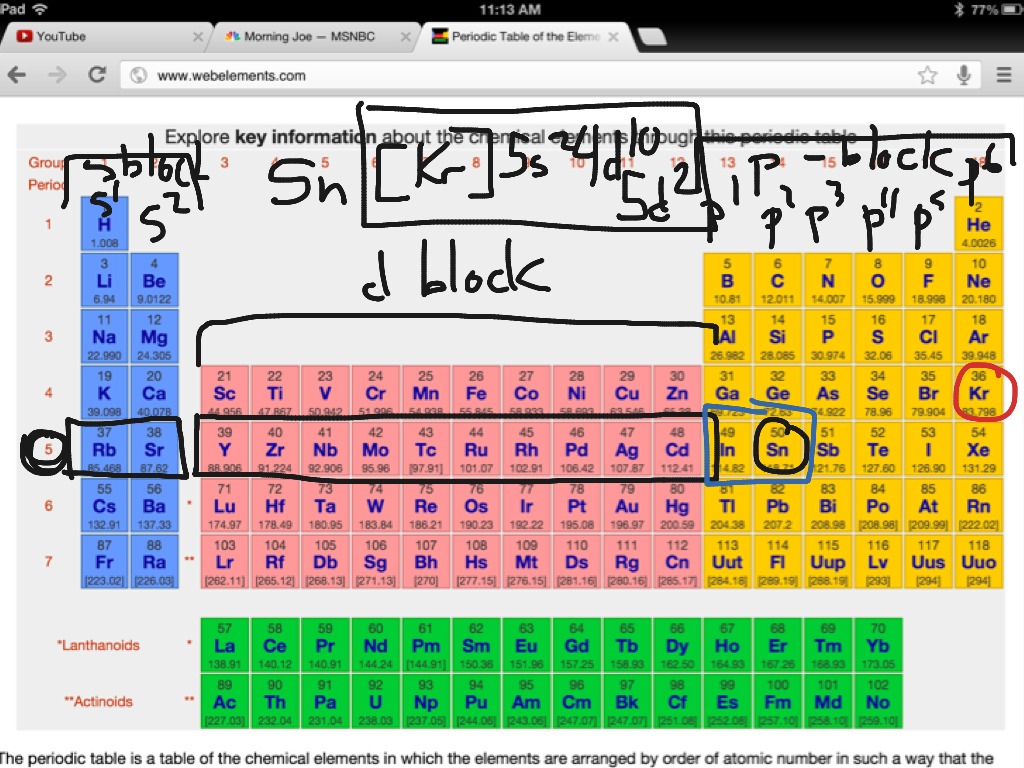
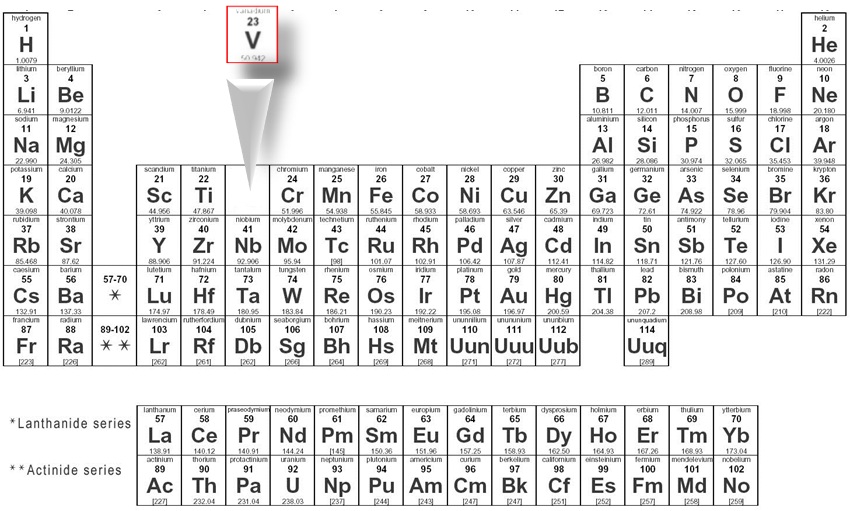
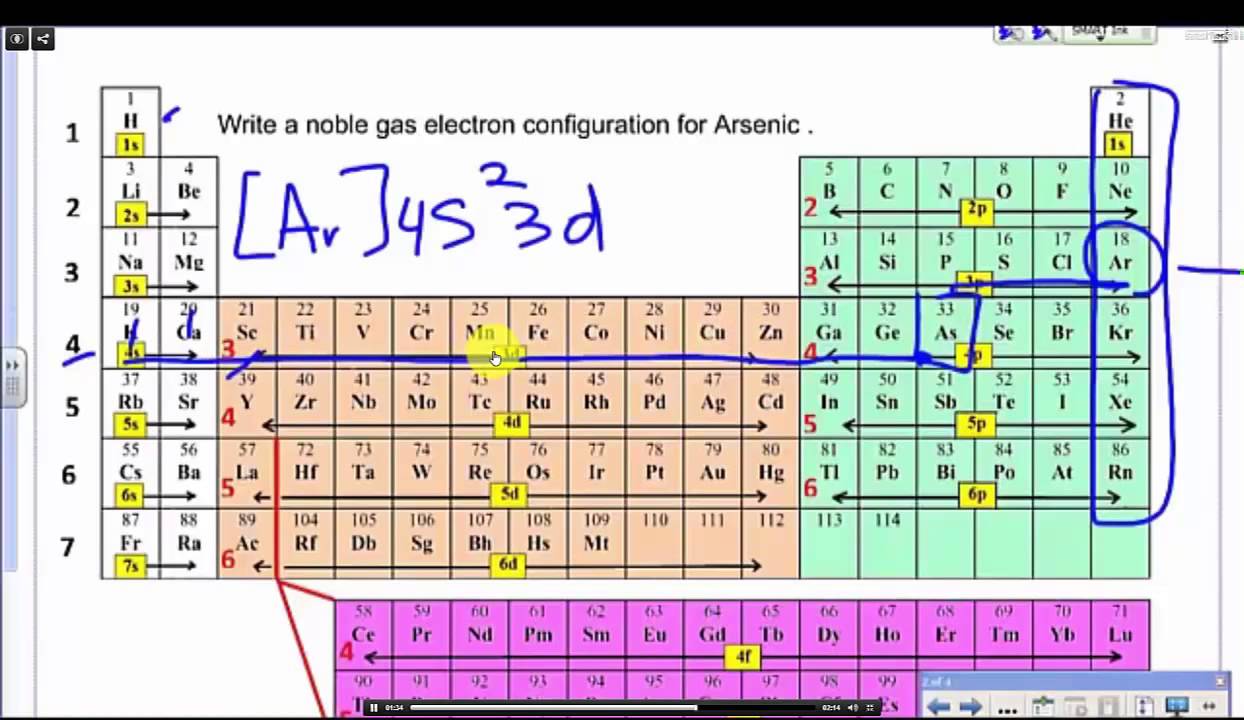
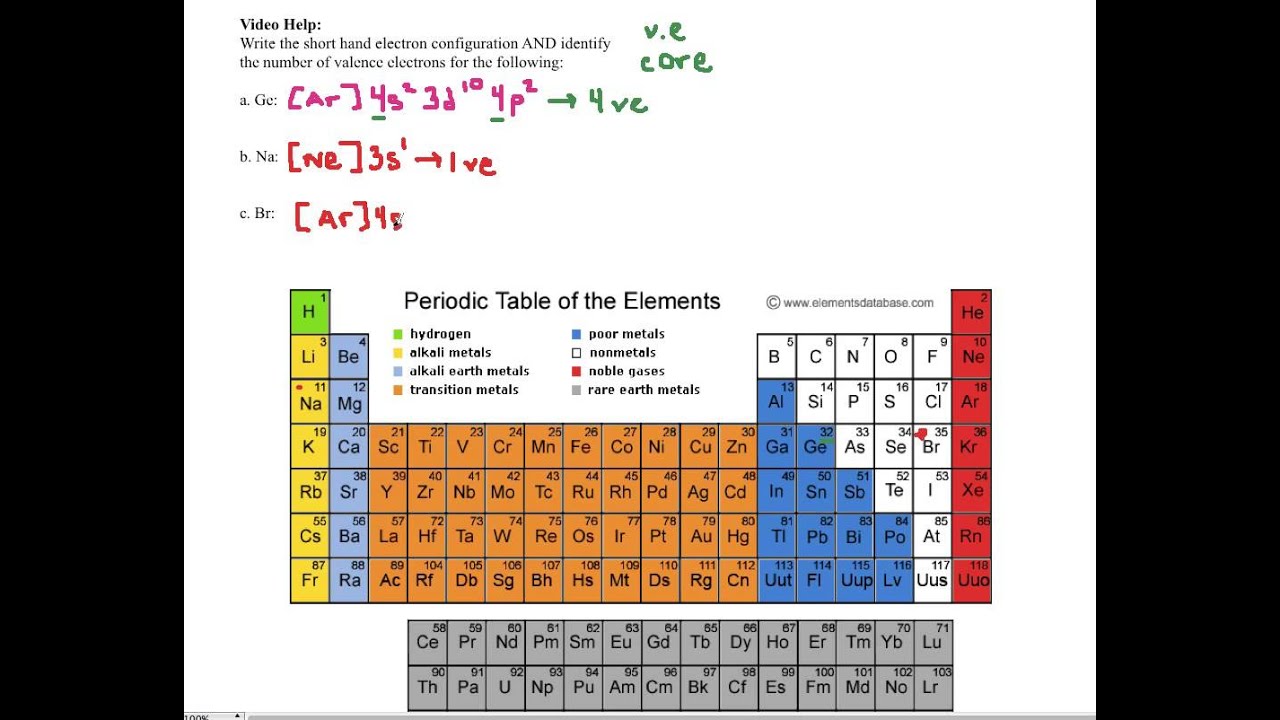
Look at the periodic table and choose the noble gas in the far right column that comes before the element youre interested in. If you need to write a configuration for a cation positive ion remember to subtract the number of electrons equal to the charge from the total number of electrons before starting. The next six electrons will go in the 2p orbital. Follow the steps below to write short cut version of electron configurations.
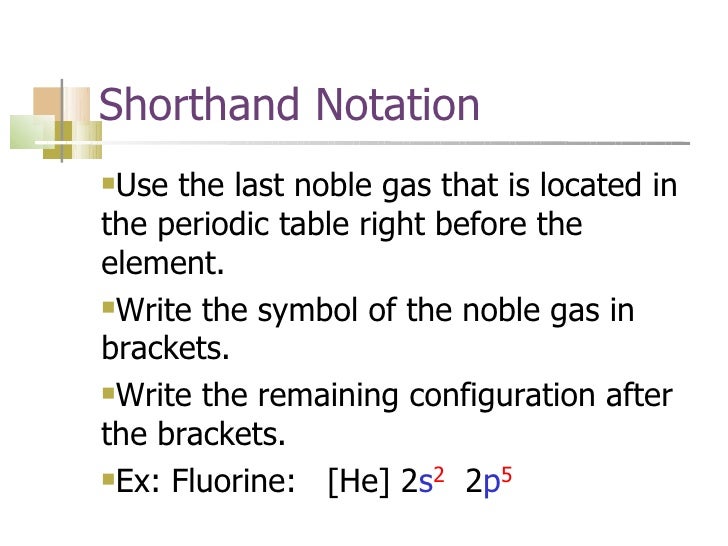
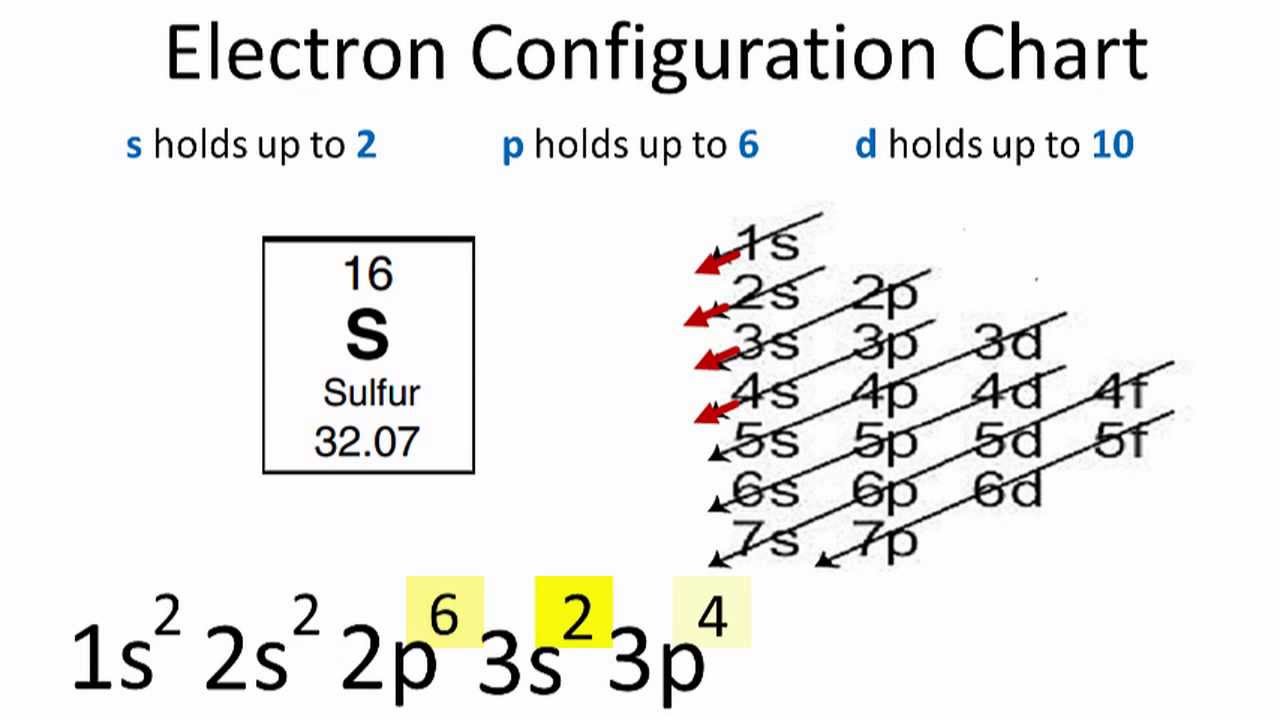
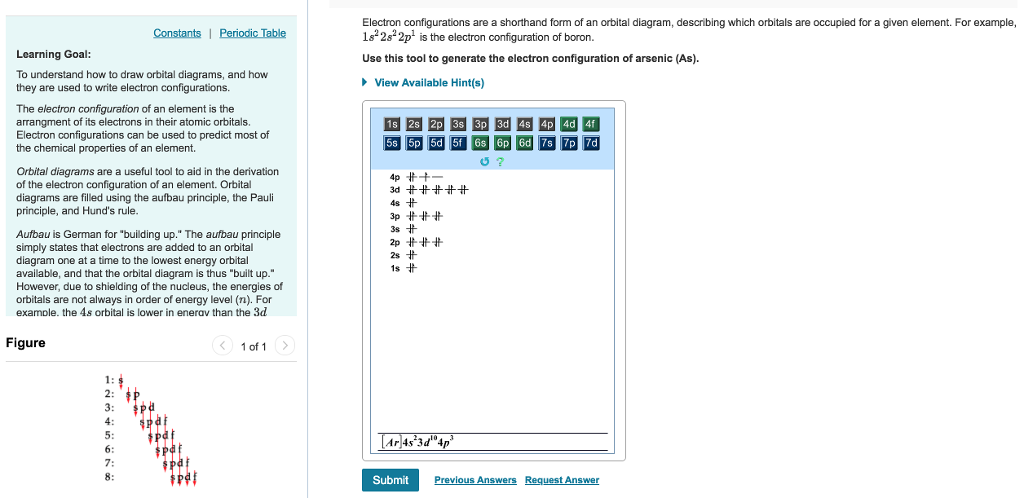
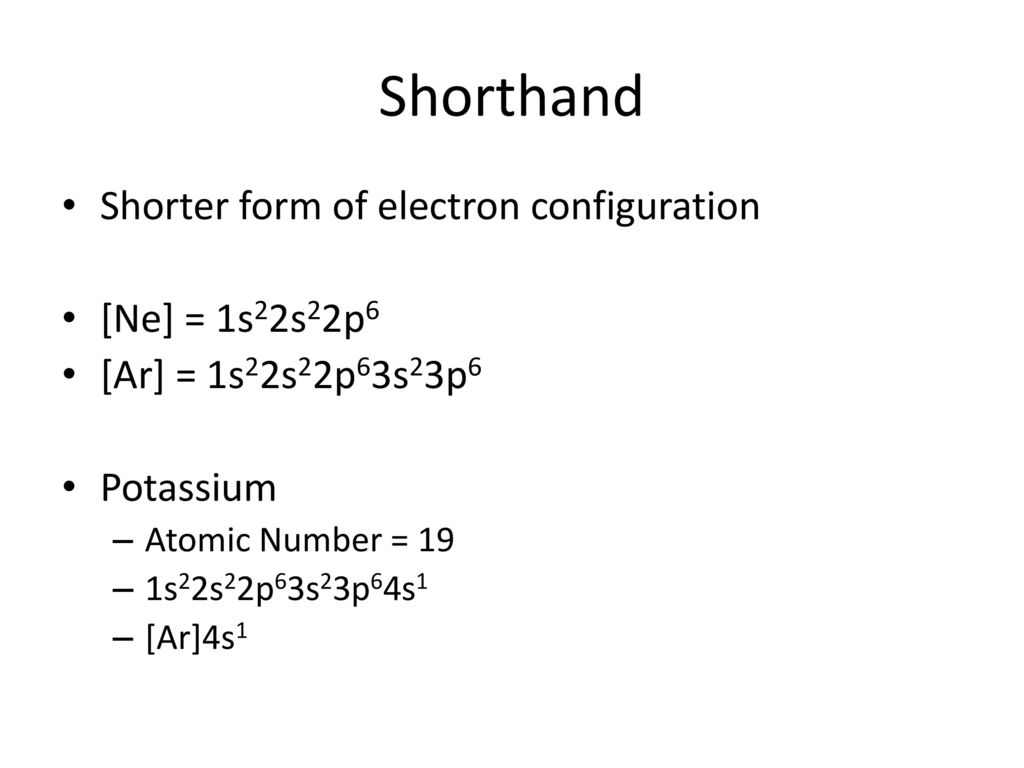
Find the element on the periodic table. The first number is the principal quantum number n and the letter represents the value of l angular momentum quantum number. Write the symbol of the noble gas in brackets to start your electron configurationput the atomic number of the noble gas beneath the symbol to let you know the number of electrons already. 1s 2 2s 2 2p 6.
Likewise if youre writing a configuration for an anion negative ion remember to add the number of electrons equal to the charge to the total number of electrons before starting. Step 1 find the symbol for the element on a periodic table. Orbital diagrams and electron configuration basic introduction chemistry practice problems duration. Follow these steps to write abbreviated electron configurations.
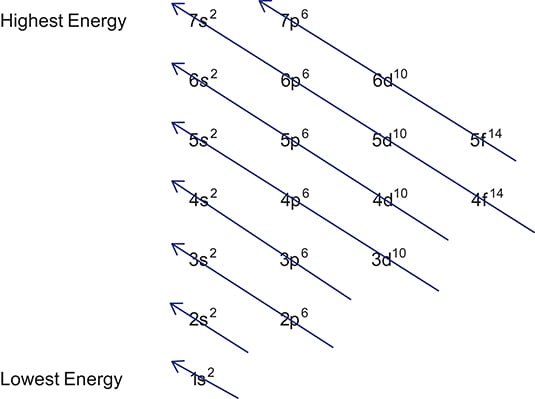
Put the chemical symbol for the noble gas in front of the configuration in square brackets and then write the configuration for any additional electrons in the standard way. The p orbital can hold up to six electrons. For example to write an abbreviated electron configuration for zinc atoms we first find zn on the periodic table see belowstep 2 write the symbol in brackets for the noble gas located at the far right of the preceding horizontal row on the table. Since 1s can only hold two electrons the next 2 electrons for iron go in the 2s orbital.
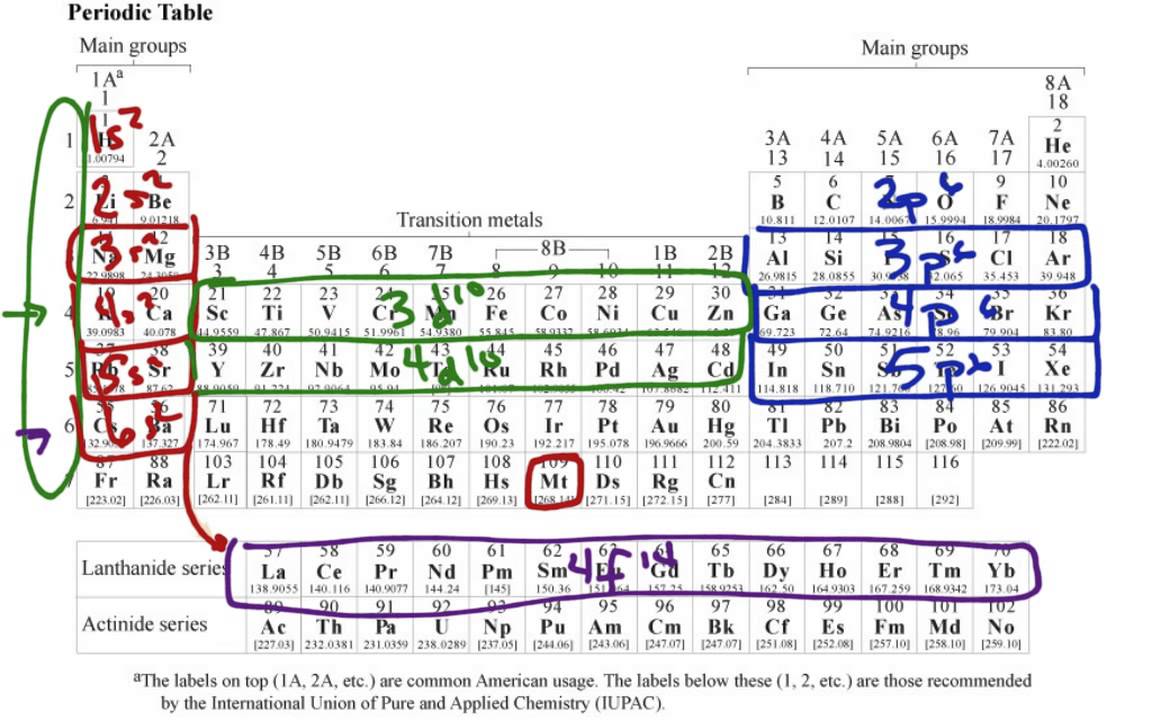
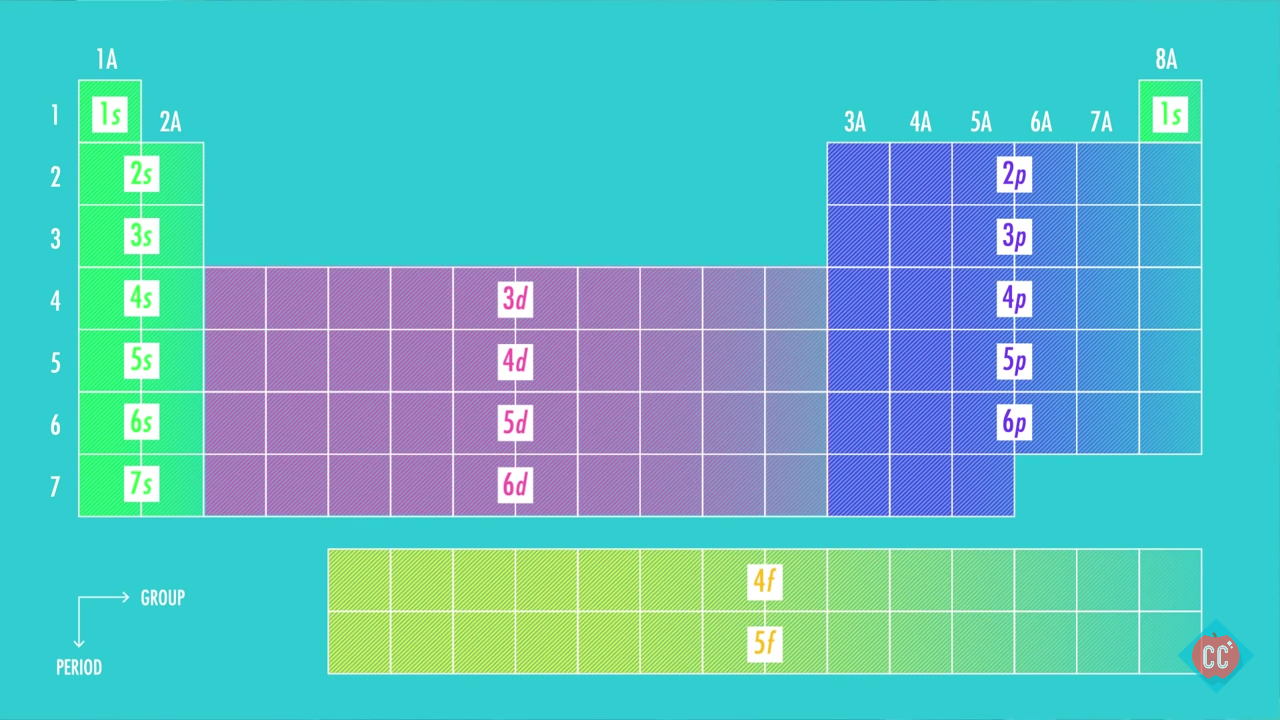
Notice numbers 1 through 8 at the base of the table.




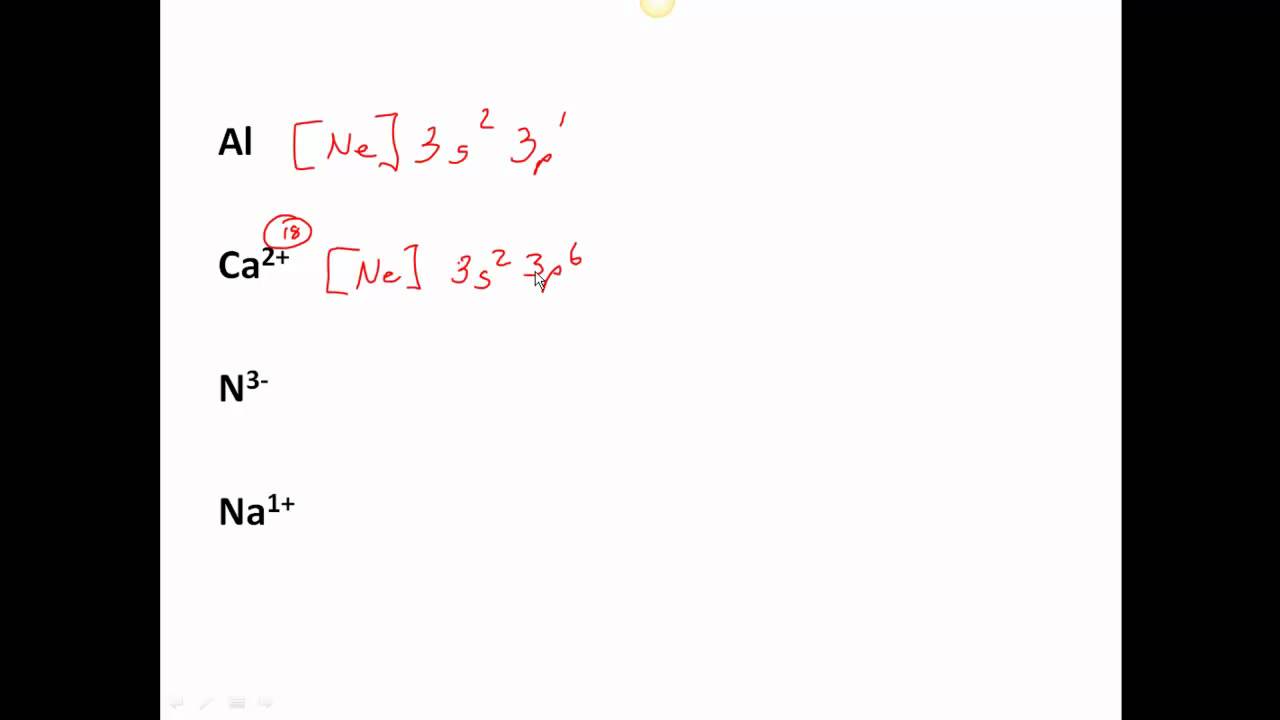



















.jpg)


















/800px-Orbital_representation_diagram.svg-589bd6285f9b58819cfd8460.png)