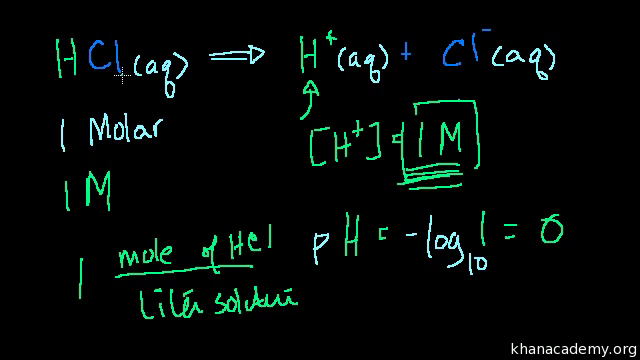How To Calculate Ph From Molarity
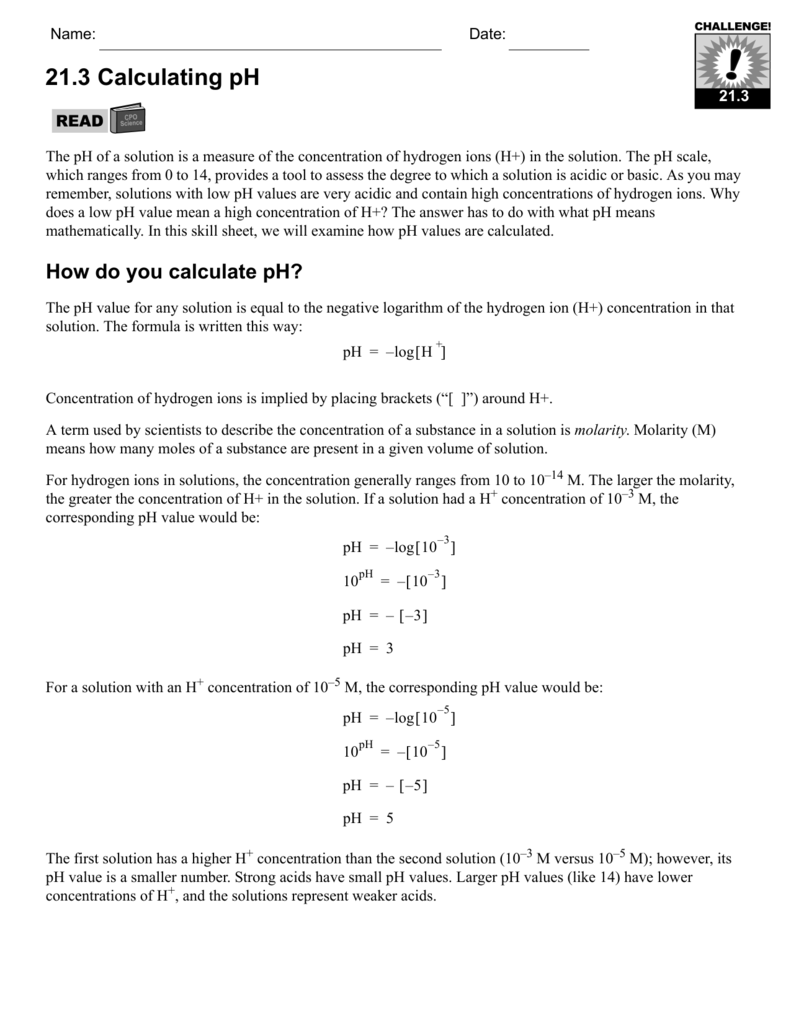
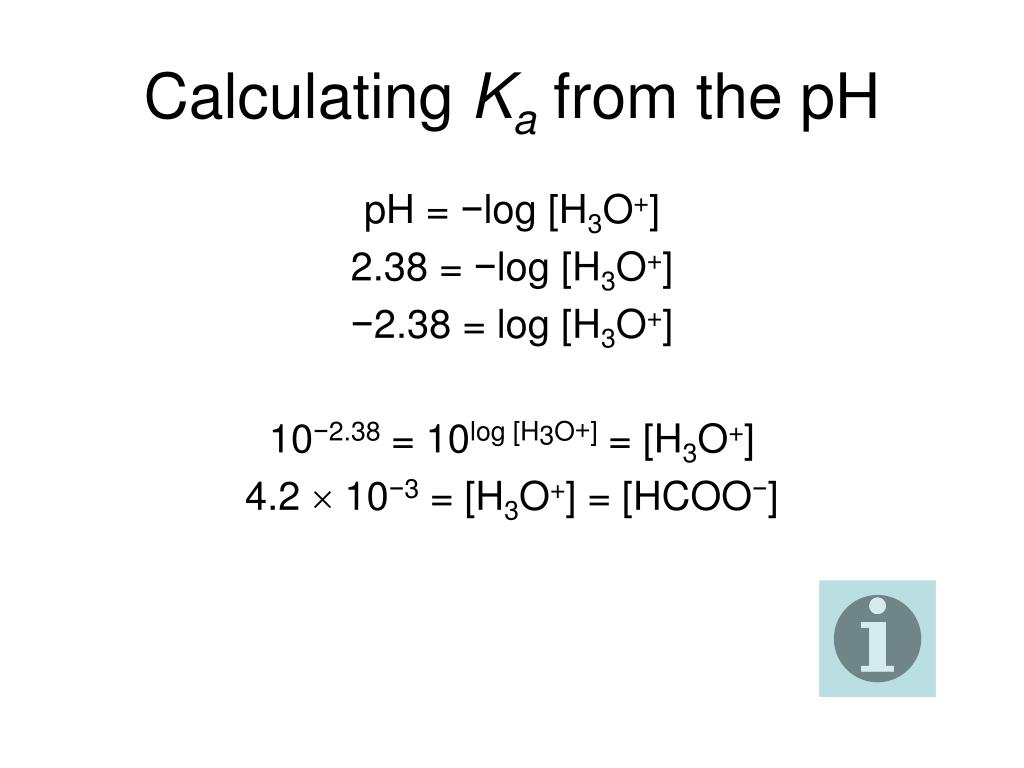
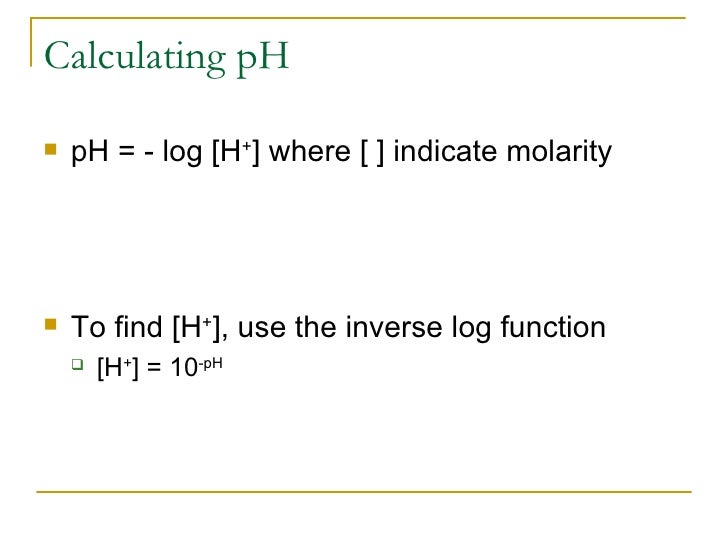
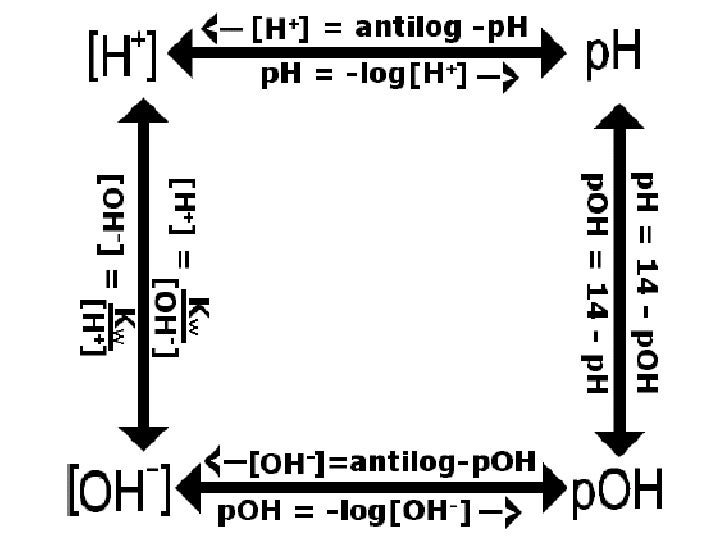
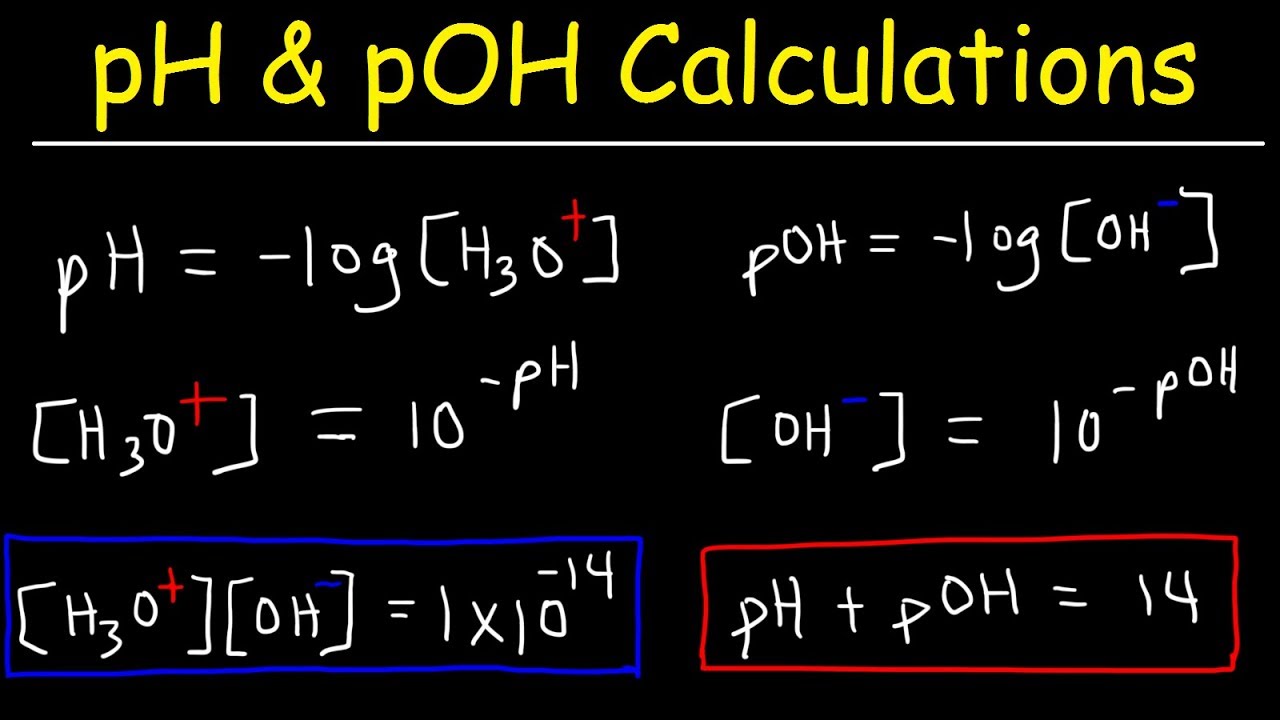
Ph logh the brackets refer to molarity m.

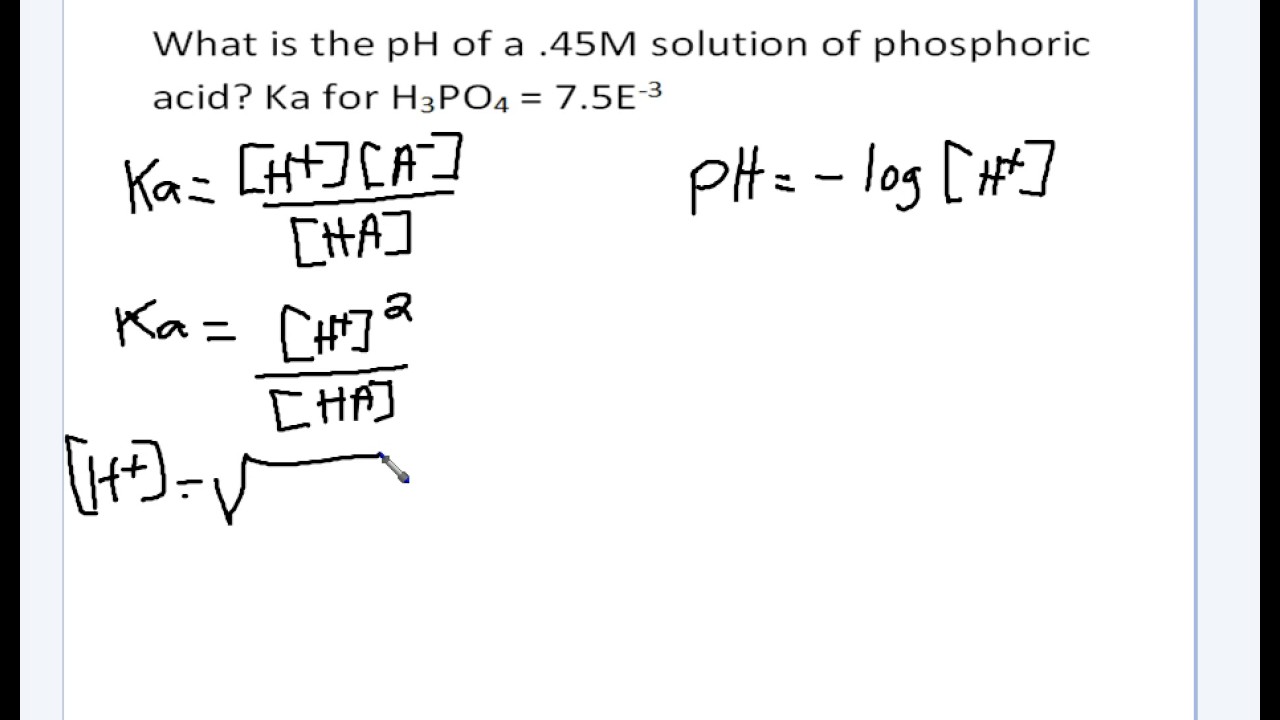
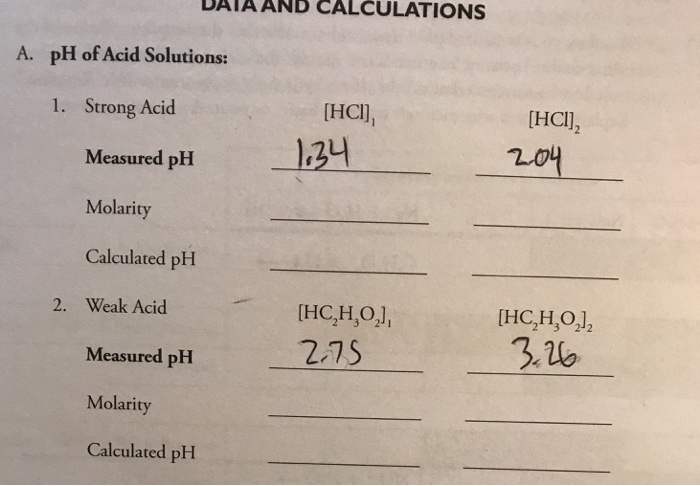
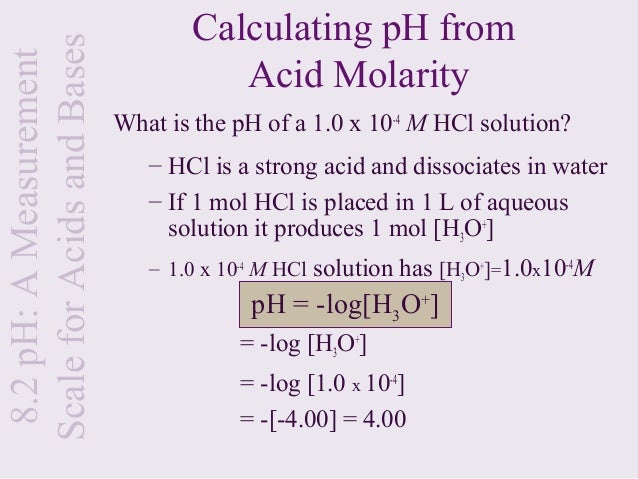
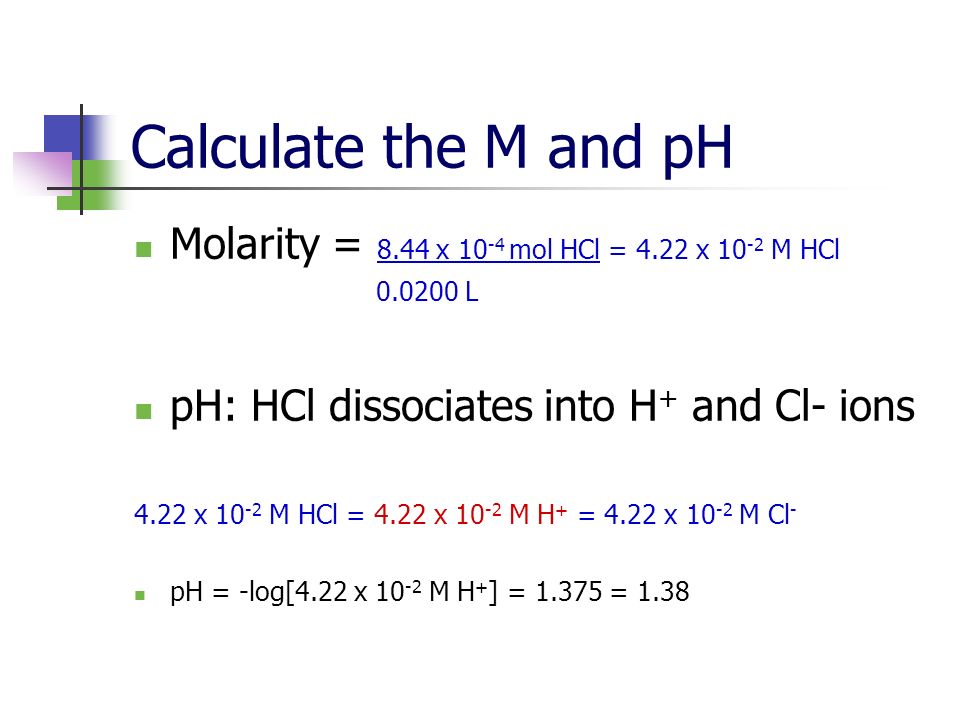
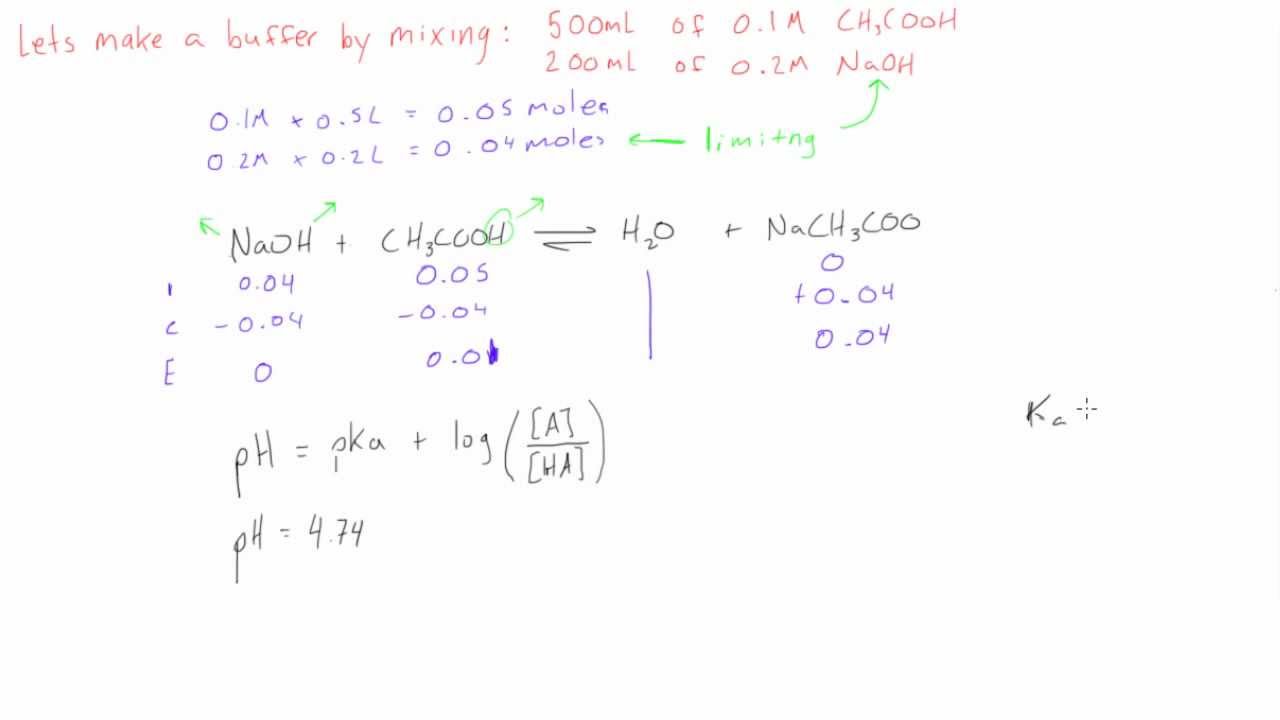
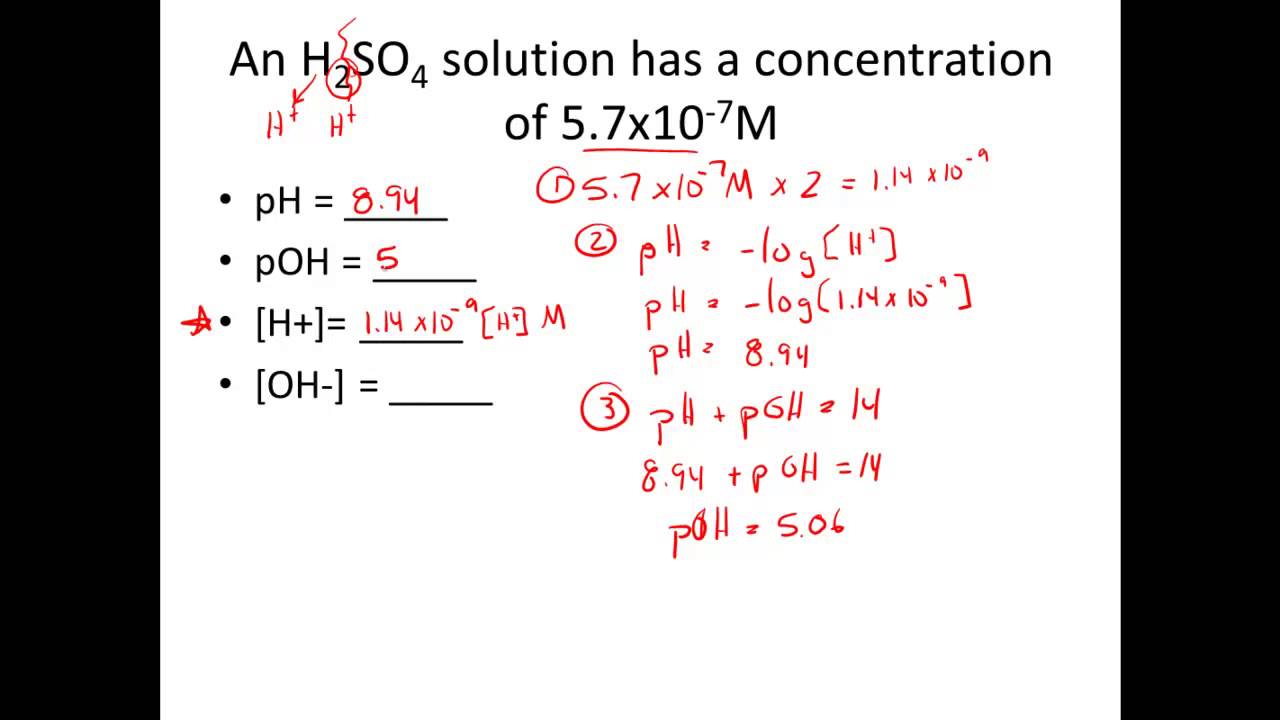
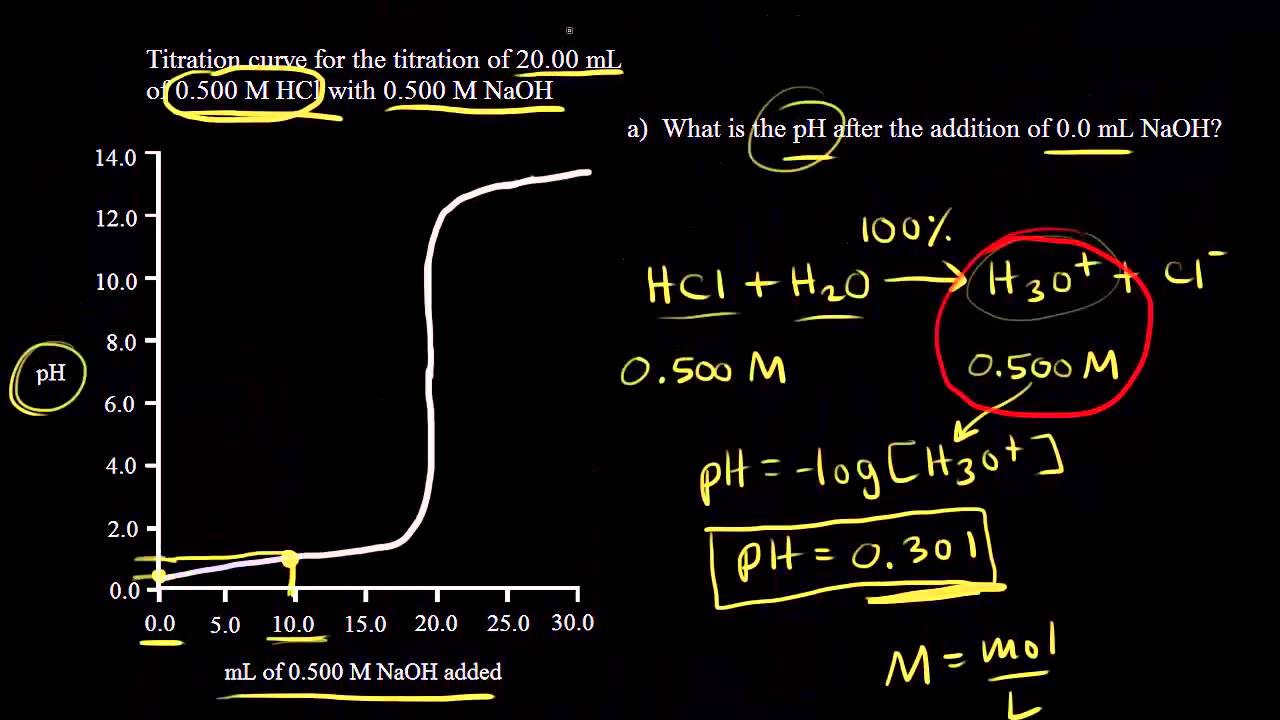
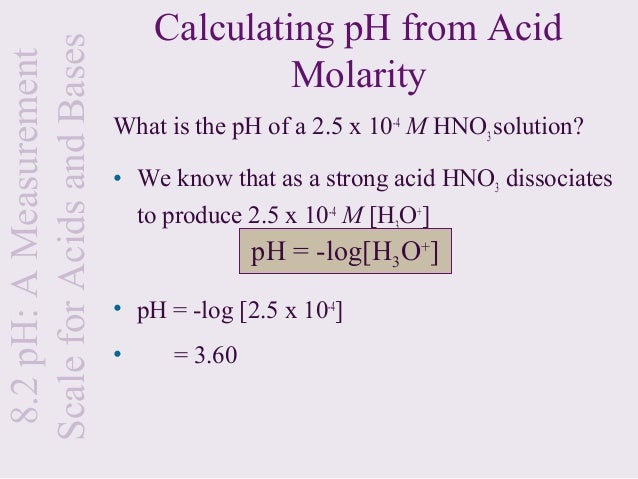
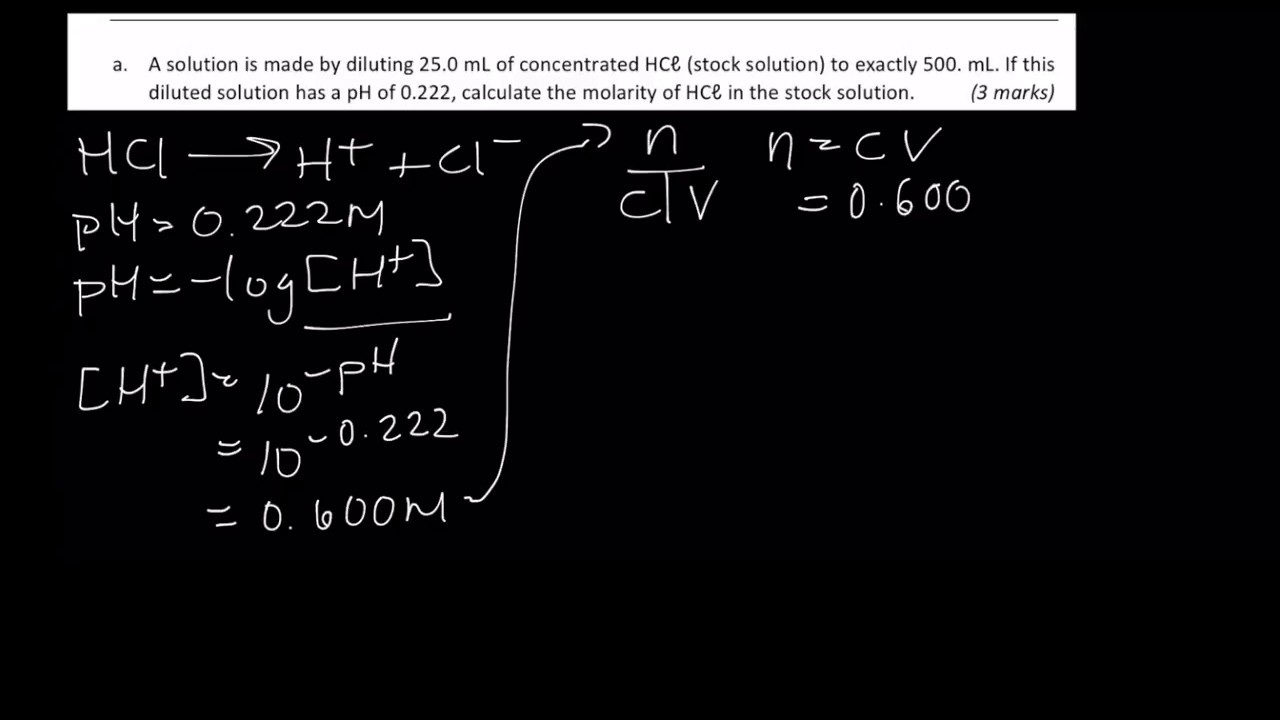
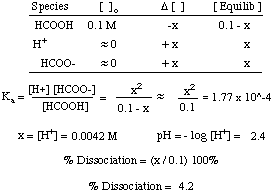
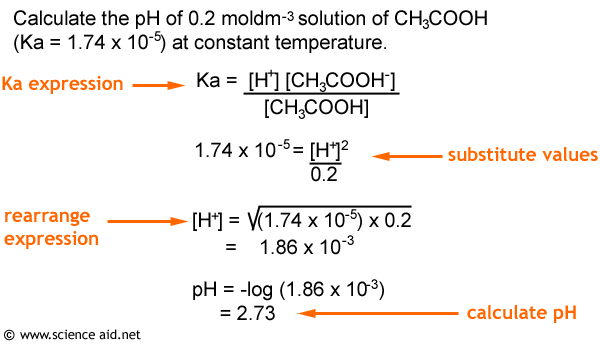
How to calculate ph from molarity. Ph log 63 10 5 42. The ph is then calculated using the expression. The hcl is a strong acid and is 100 ionized in water. The formula to calculate ph is.
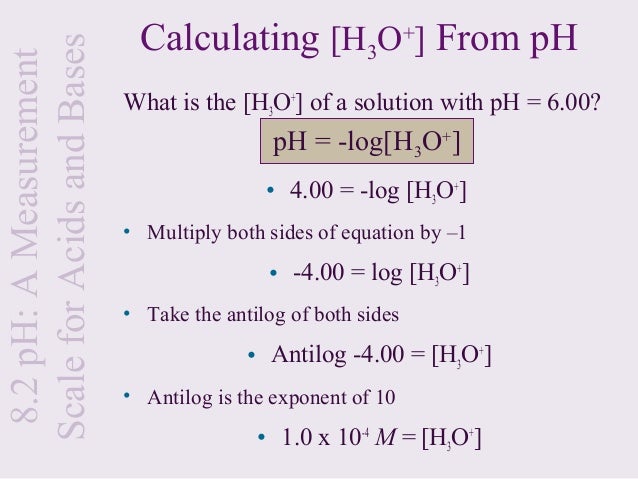
To calculate it take the log of a given hydrogen ion concentration and reverse the sign. To go from molarity to ph use your calculator or a similar tool to take the logarithm to the base 10 the default base of the molarity reverse the sign to get a positive value and youre done. This means our h3o equals 10 2 or 001m. Hcl h2o h3o cl we measure a ph of 2.
The formula for ph is ph logh. Review of acids bases and ph formula. Ph log h3o. We take for example hcl.
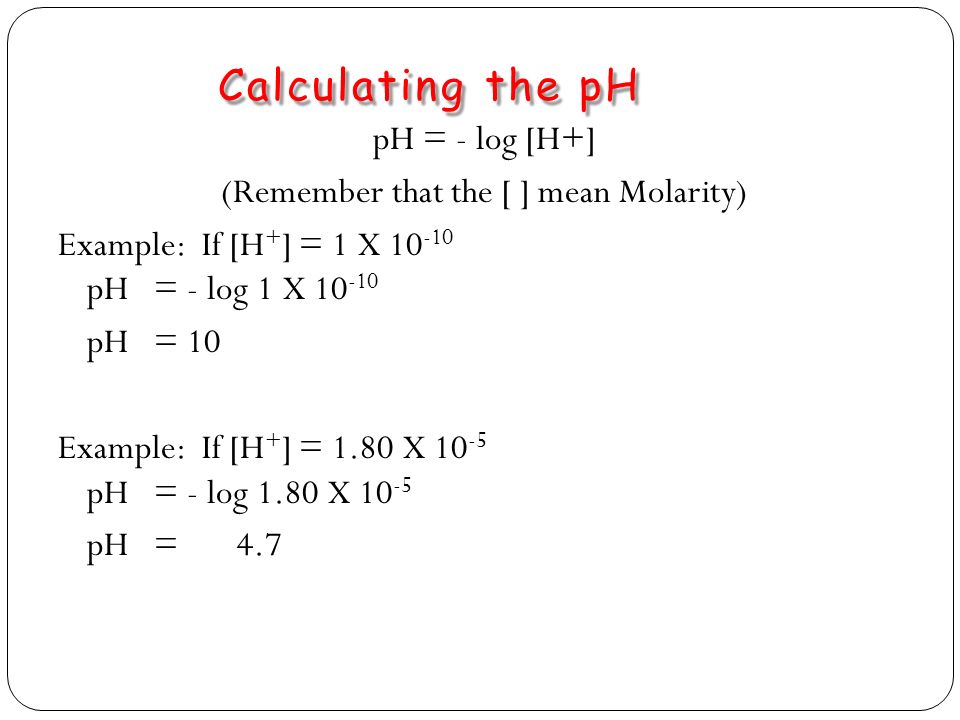
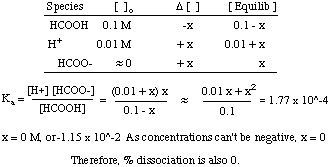
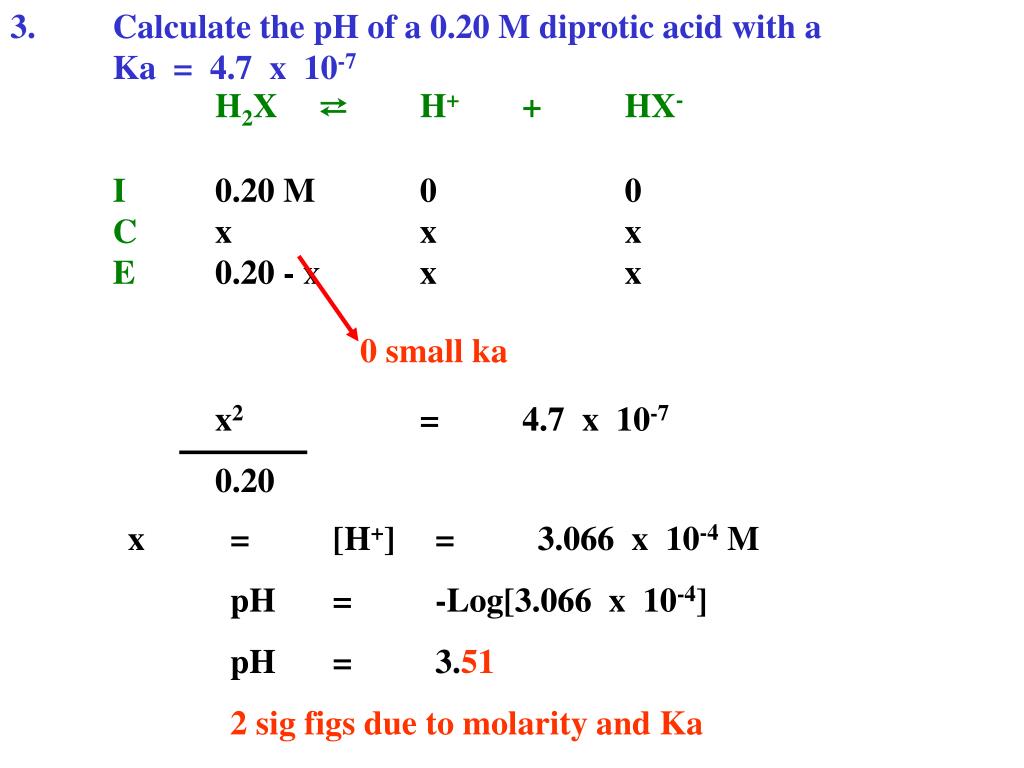
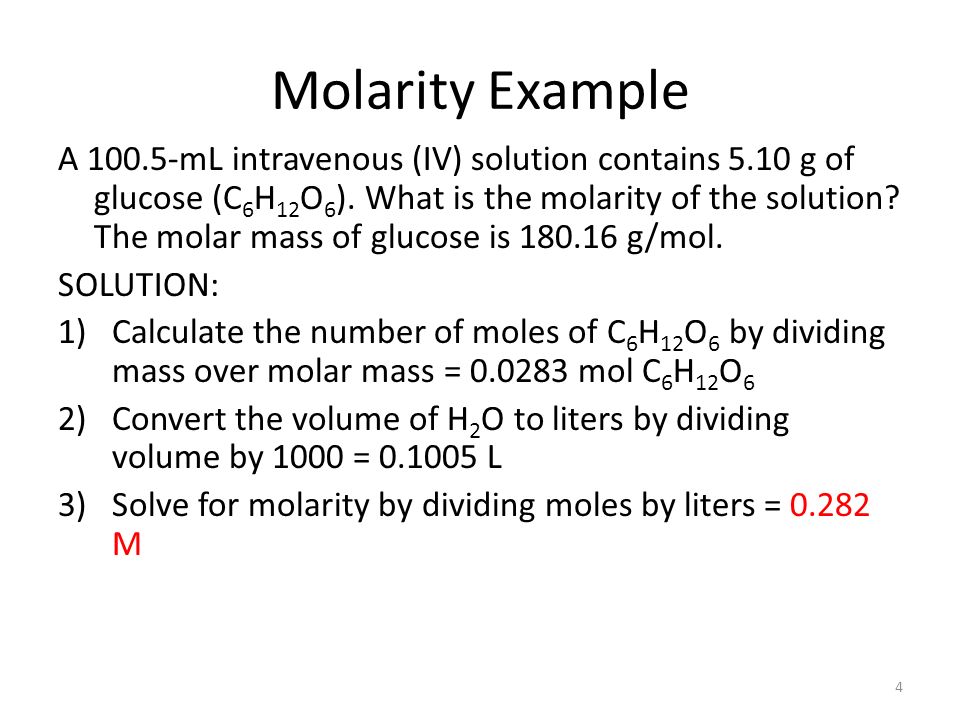
To calculate it take the log of the hydrogen ion concentration and reverse the sign to get the answer. Find the ph of a 00025 m hcl solution. If the molarity of an aqueous solution is 63 10 5 m what is the ph. To calculate the ph of an aqueous solution you need to know the concentrationof the hydronium ion in moles per liter molarity.
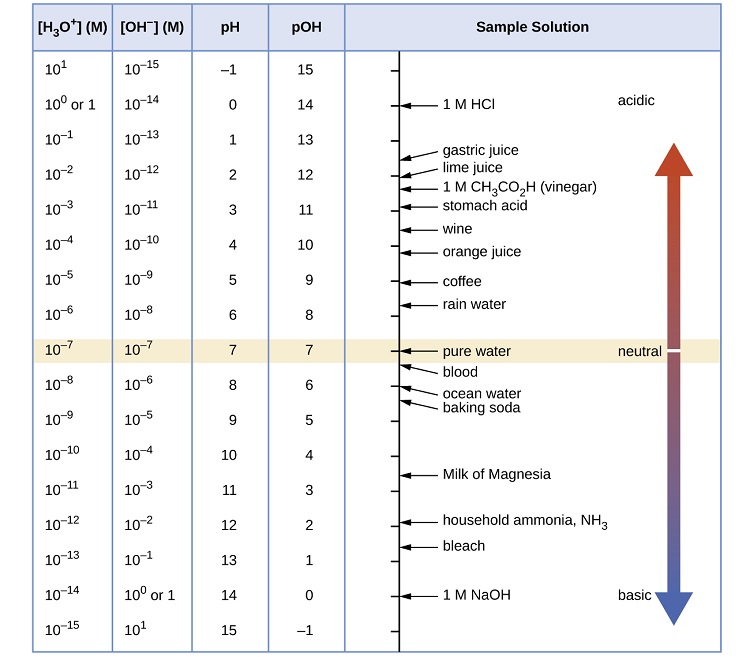
The phscale runs from 0 to 14a value of seven is considered neutral less than seven acidic and greater than seven basic. Molarity is given in units of moles per liter of solution. This means ph is the negative base 10 logarithm log on a calculator of the hydrogen ion concentration of a solution. Here we are looking for h so the best equation would h 2nd log ph since we already have the value of ph 2.









/how-to-calculate-ph-quick-review-606089_final-165915b0177b4f6e82843f25097f51df.png)









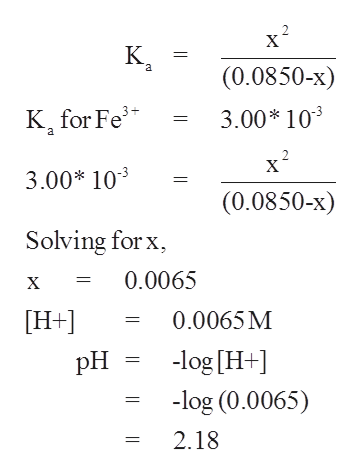

.PNG)










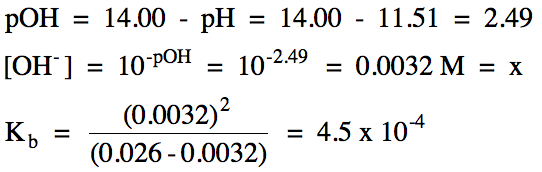


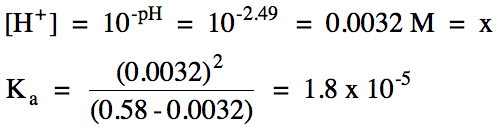






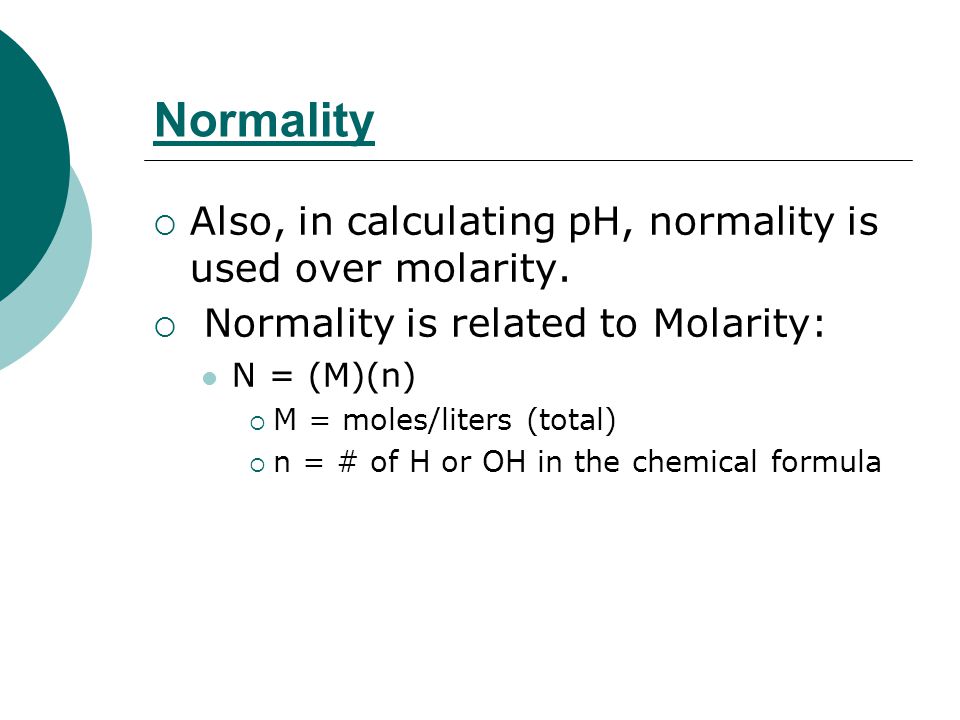

/how-to-calculate-normality-609580final2-0d5efa5a961f4fa0a7efc780921faee1.png)