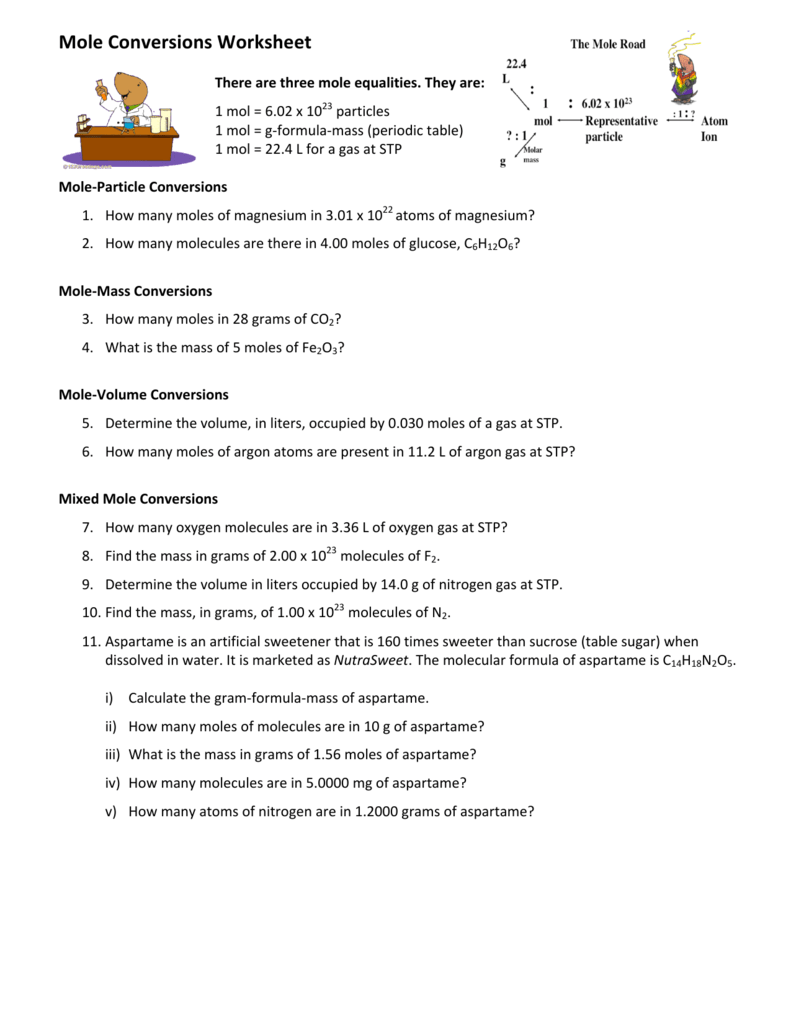
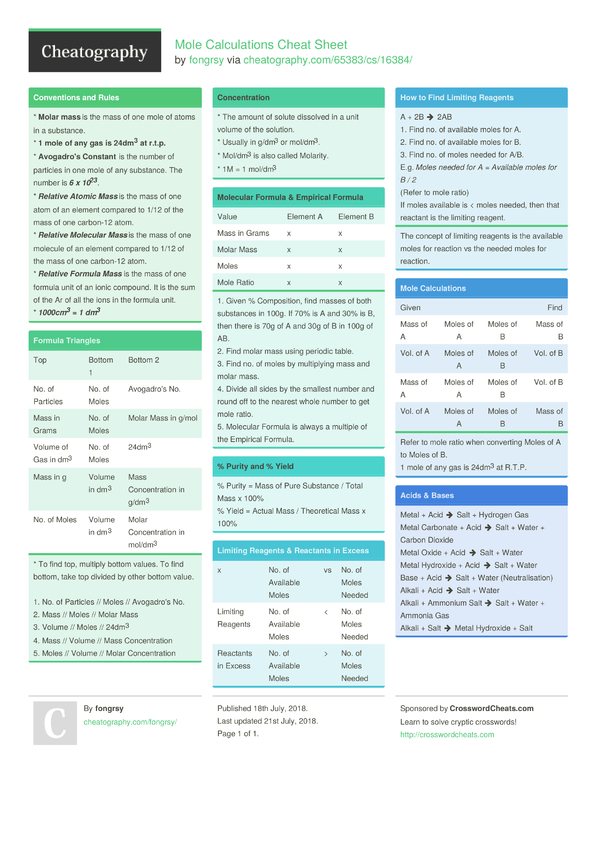
How To Calculate Moles From Grams And Volume
Converting from moles to mass grams.

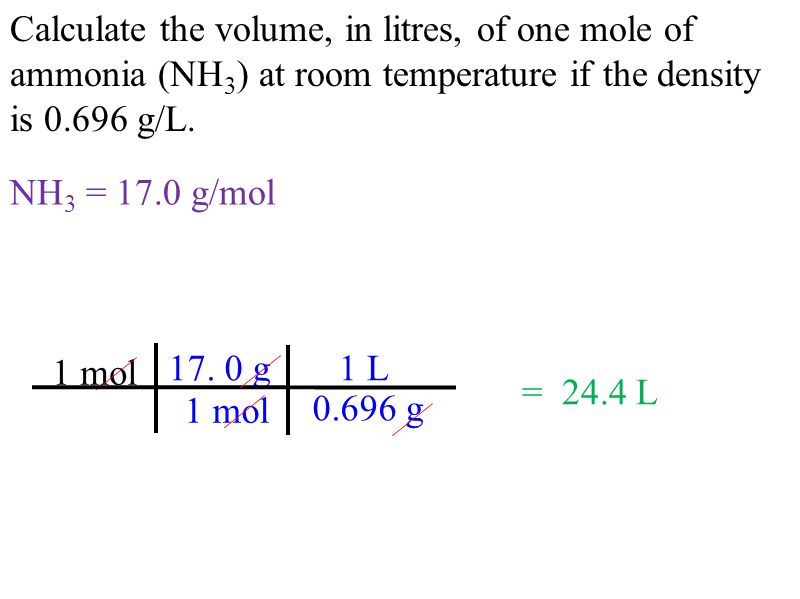
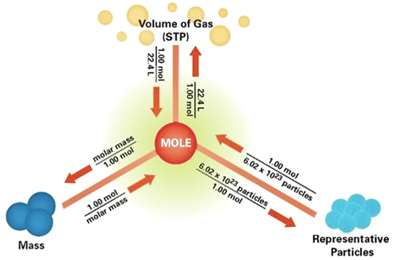
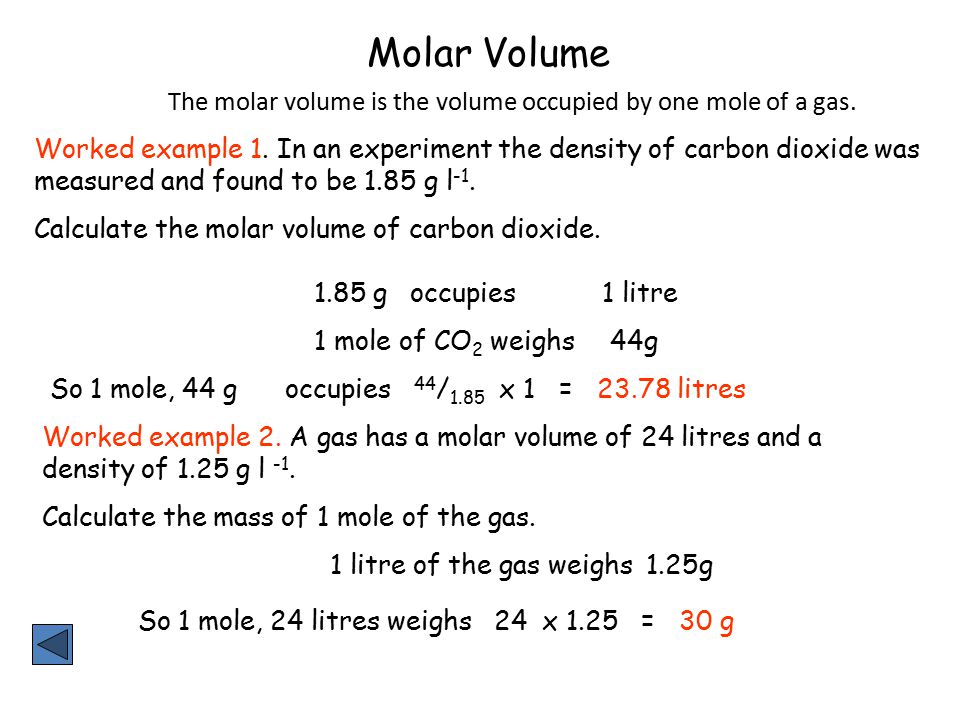
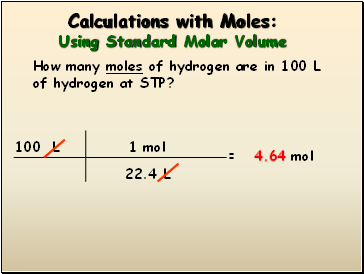
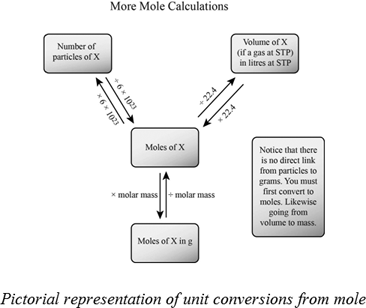
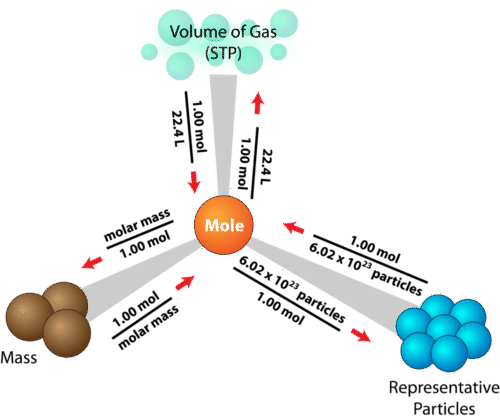
How to calculate moles from grams and volume. Converting from volume liters to moles. Calculate the volume of carbon dioxide gas co 2 occupied by a 5 moles and b 05 moles of the gas occupied at stp. Find the molar mass of. Converting from moles to volume liters.
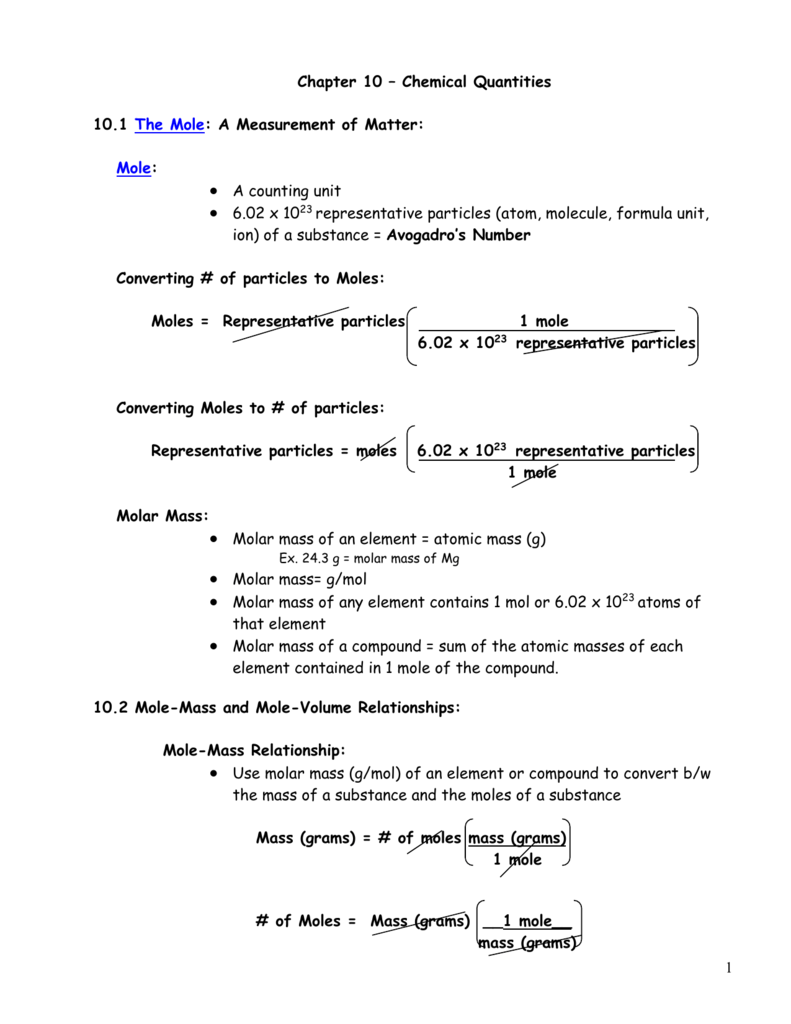
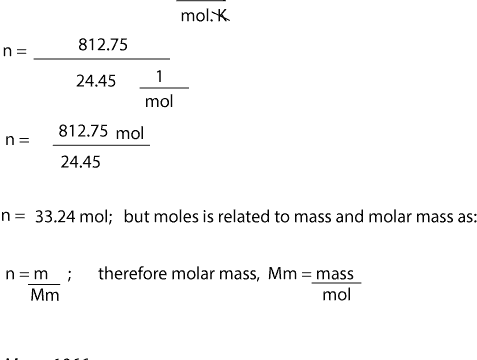
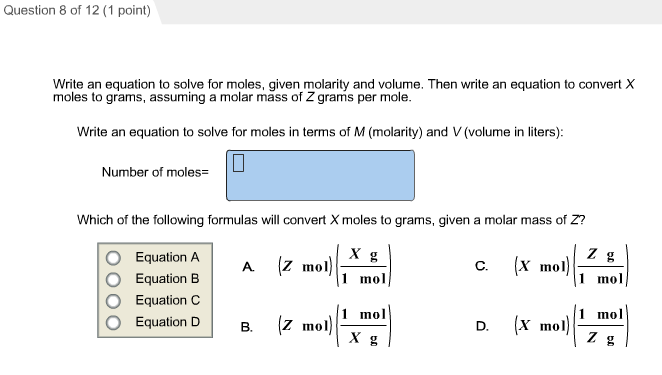
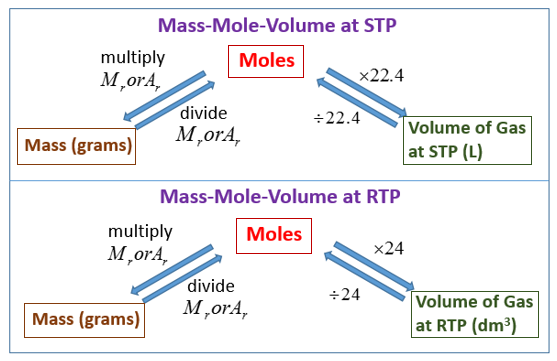
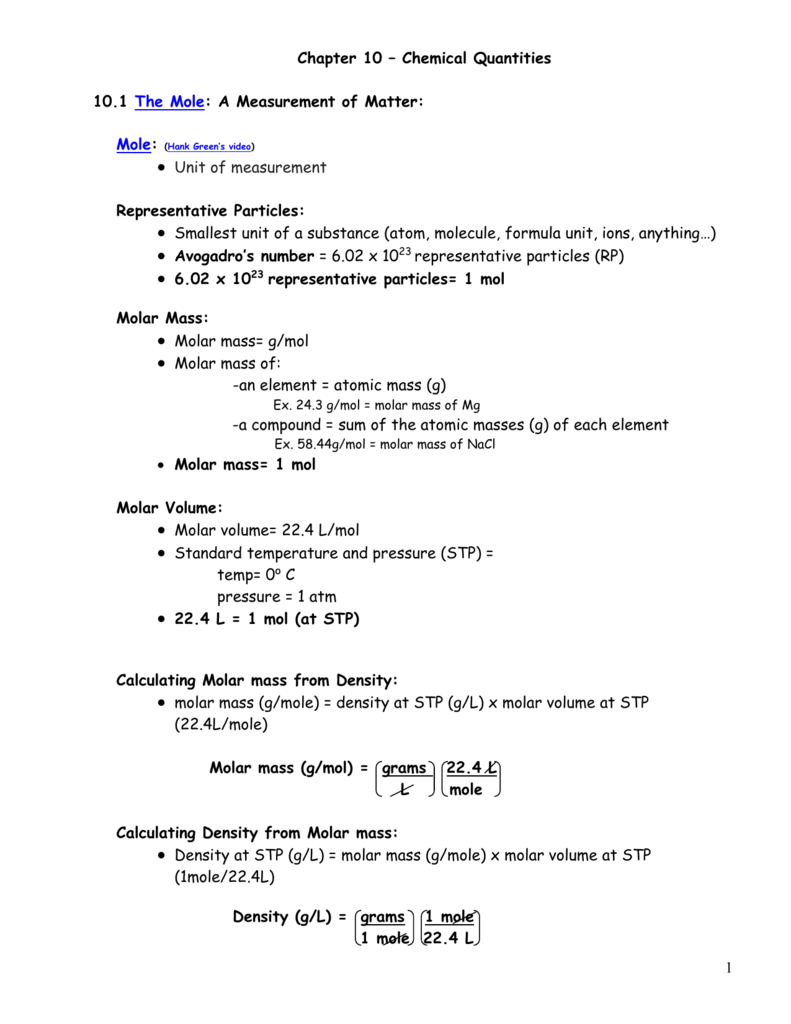
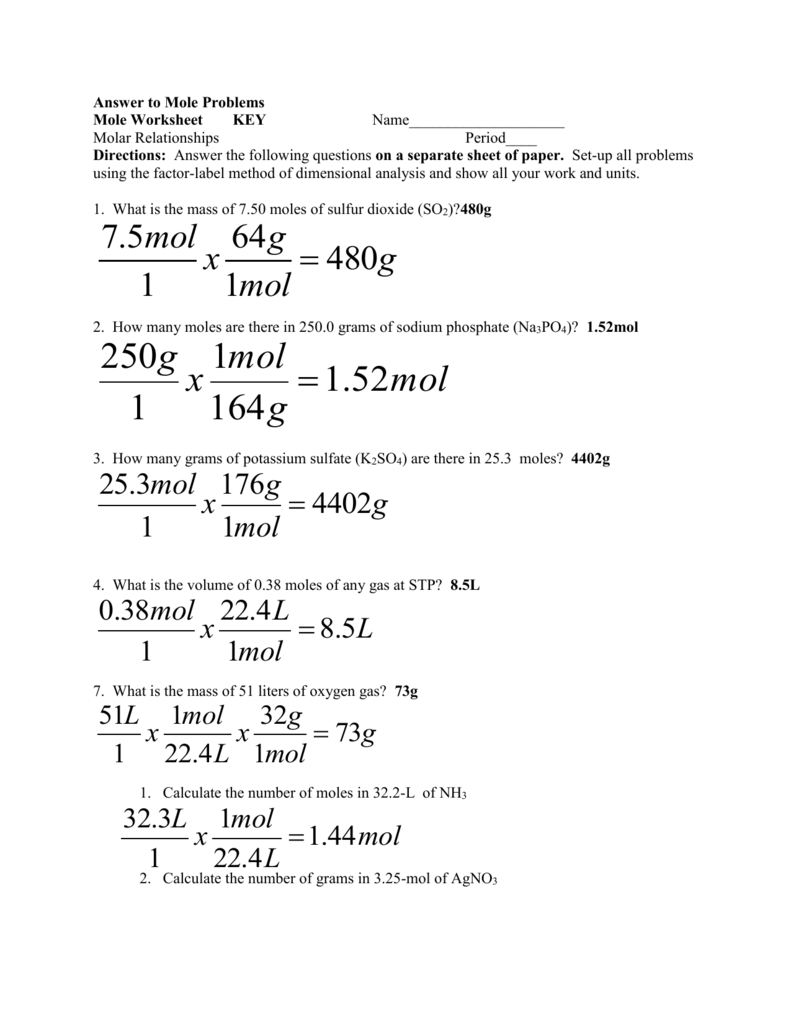
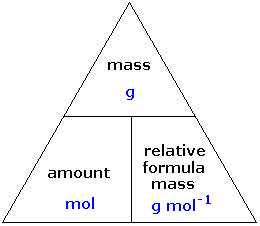
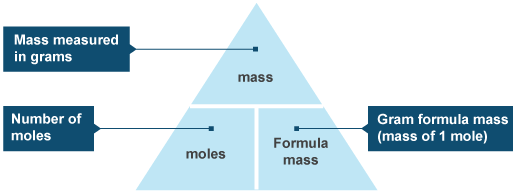
Mass of the compound molecular weight of the compound. If you dont know the mass of the solute weigh it on a scale and record the value. We can rearrange this equation to get the number of moles. Multiply your mole value by the molar volume constant 224l.
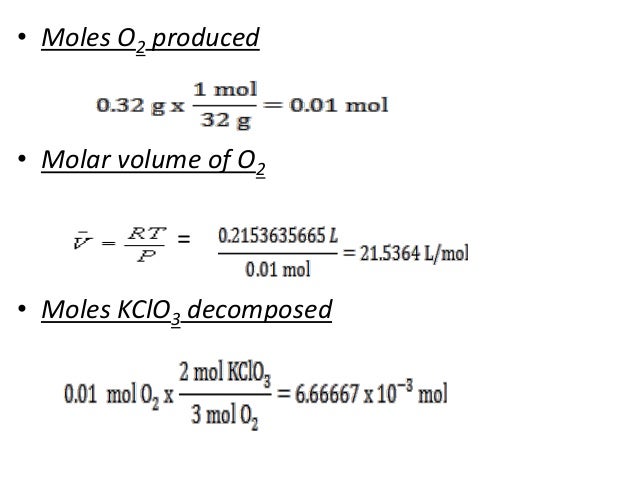
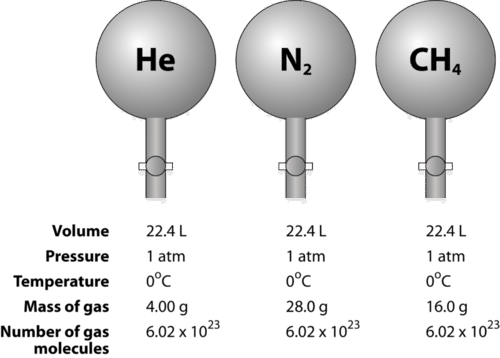
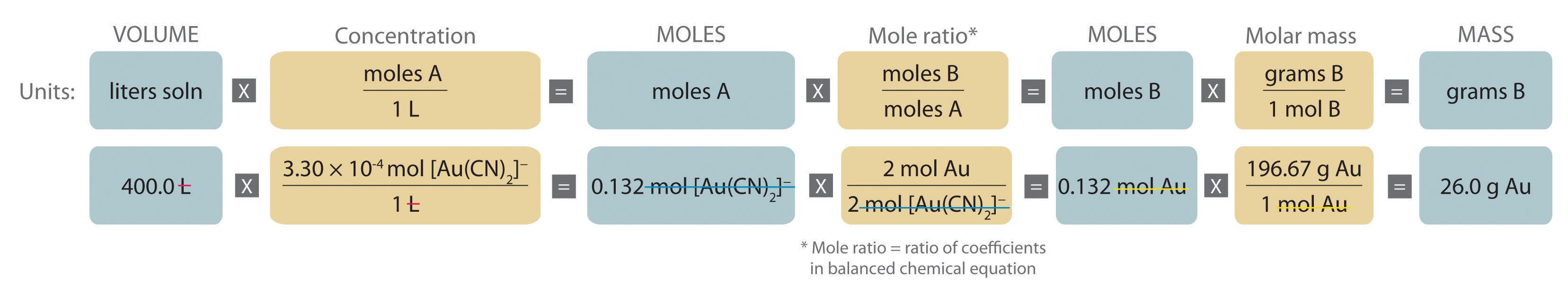
Gas volumes from moles and grams. From the periodic table the molar masses of the compounds will be extracted. Find moles by dividing given mass to molar mass find volume by multiplying number of moles to molar volume. Take the moles you got and divide it by the amount of moles in the solution to get volume v molesmolarity.
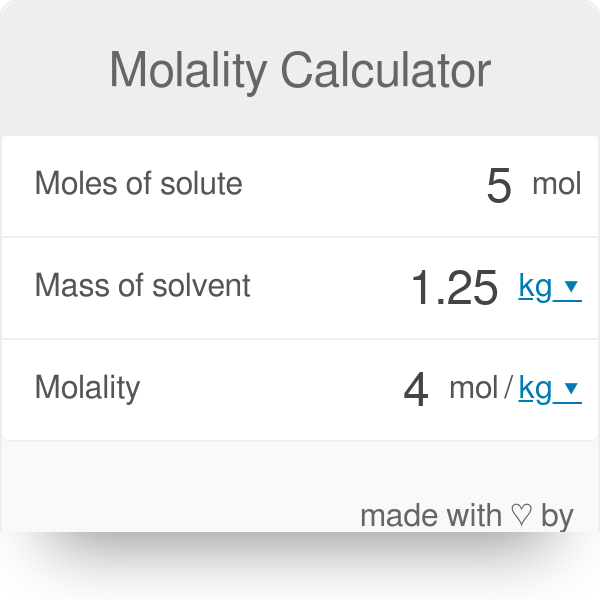
Divide your initial volume by the molar volume constant 224 l. How to calculate the number of moles. The number of moles of solute mass of solute molar mass of solute where mass is measured in grams and molar mass defined as the mass of one mole of a substance in grams is measured in gmol. From molesliter you can calculate mass in grams by converting the moles to grams using molar mass and then use the volume to calculate the final mass in that volume.
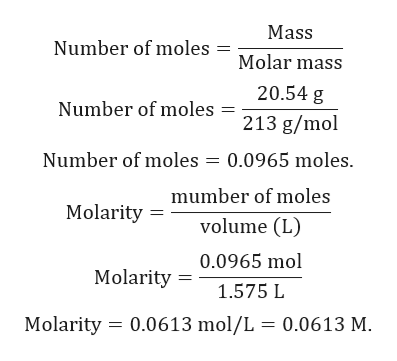
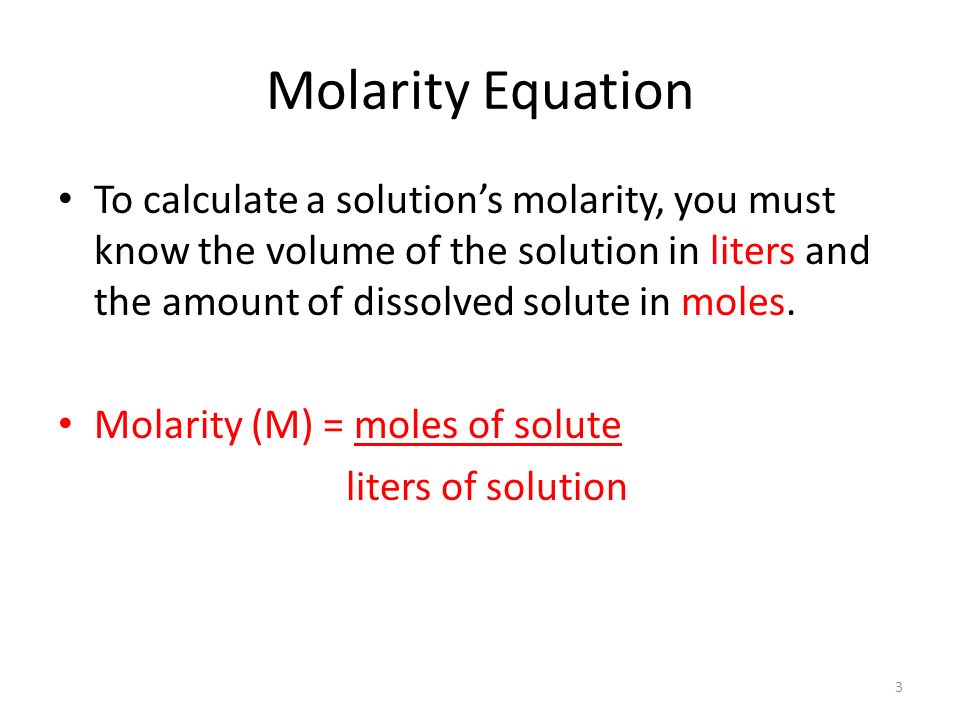
Know the basic formula for calculating molarity. To convert grams to moles the molecular weight of the solute is needed. Find moles by diving given volume to molar volume. A volume of co 2 number of moles of co 2 224 l 5 224.
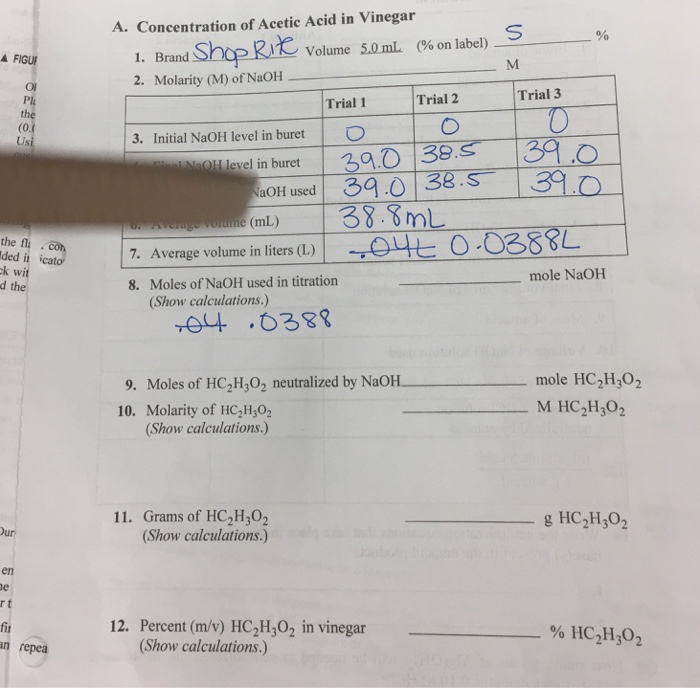
Molarity is the number of moles of a substance in one litre of solution. Take the grams divide by molar mass to get moles moles massmolar mass 2. The official symbol for molarity is c concentration but many people use the old symbol m. Calculating molarity with mass and volume 1.
Finding molarity demands that you have the number of moles and the number of liters. M nv where n is the number of moles and v is the volume in litres. Molar mass of k 391 g. And these are steps to convert from liters of a gas to grams of a gas.
The number of moles represents the fraction.

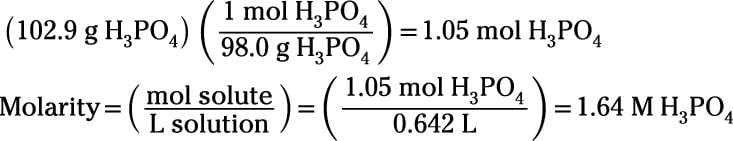


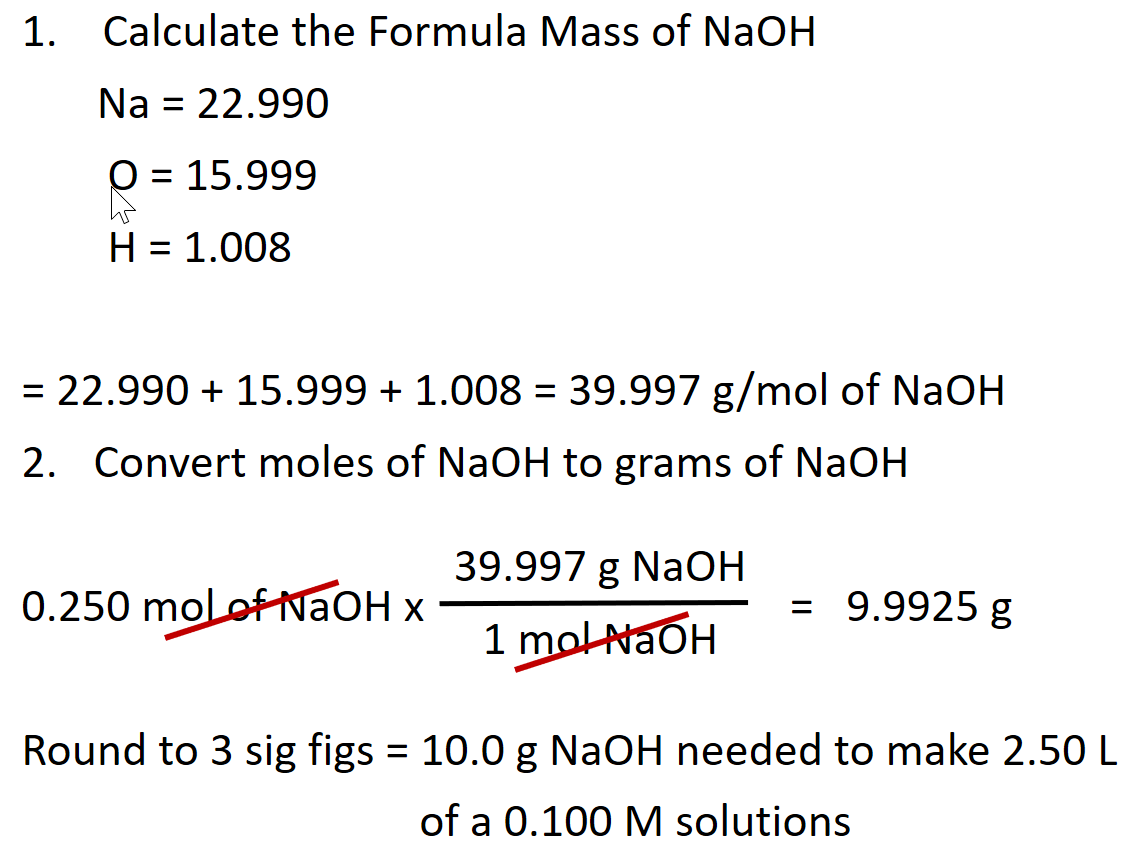





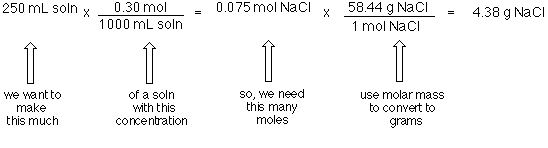



.PNG)







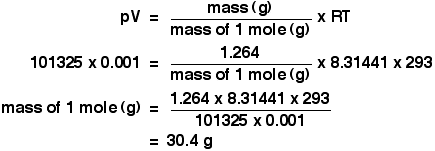








.PNG)









/mass-percent-composition-example-609567_V2-01-89c18a9d30ea43b494d09b81f7ffefc1.png)
.PNG)








/606823-calculate-molarity-of-a-solution-FINAL-5b7d7e15c9e77c0050355d4e.png)



.PNG)



/how-to-calculate-normality-609580final2-0d5efa5a961f4fa0a7efc780921faee1.png)