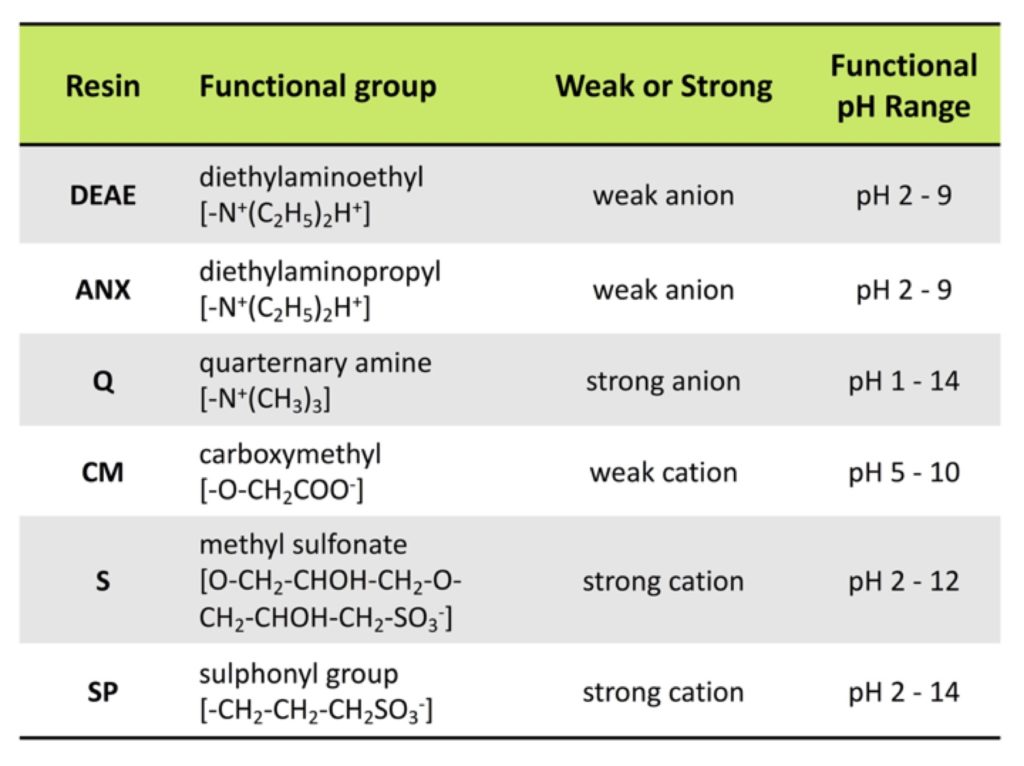
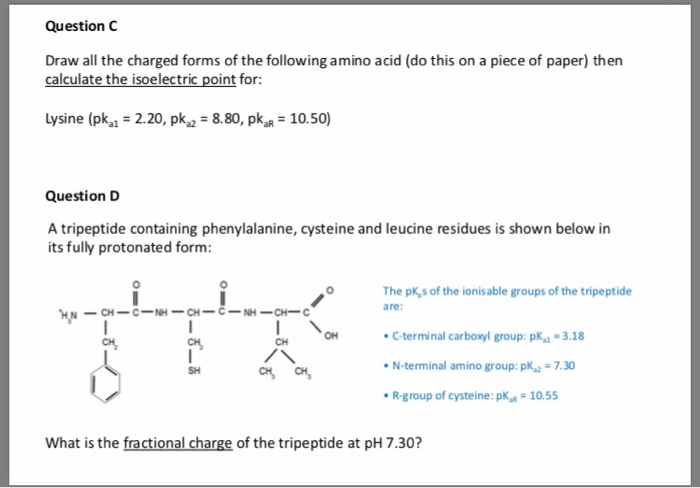
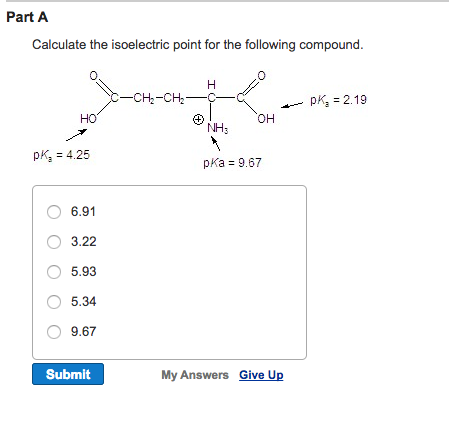
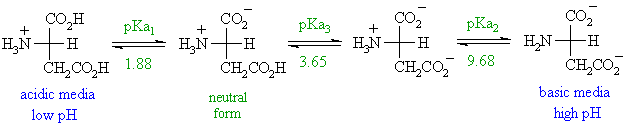How To Calculate Isoelectric Point
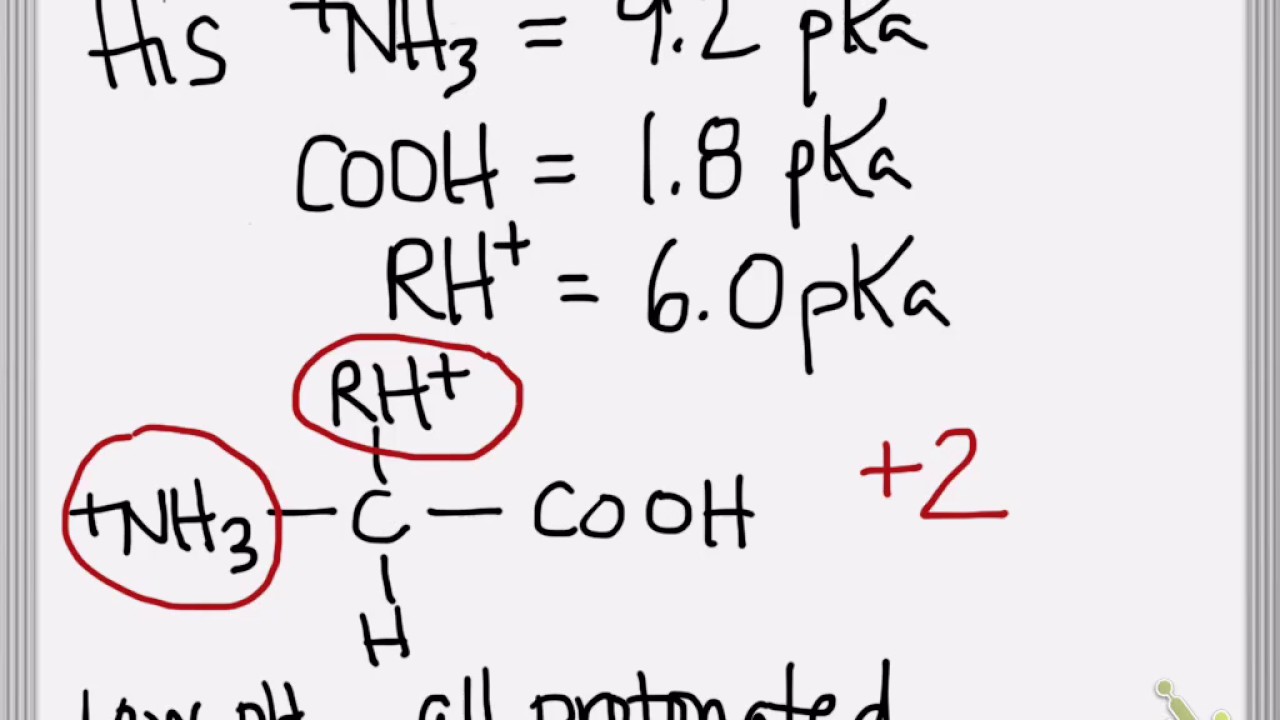
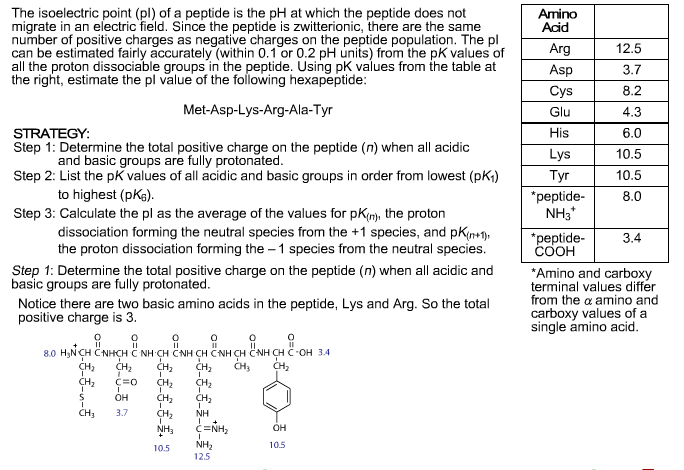
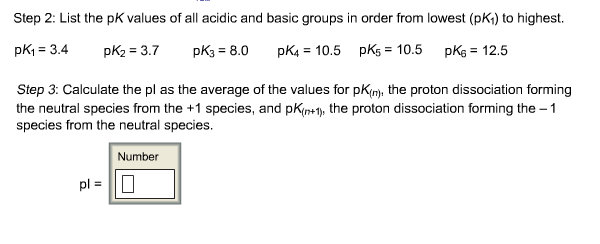
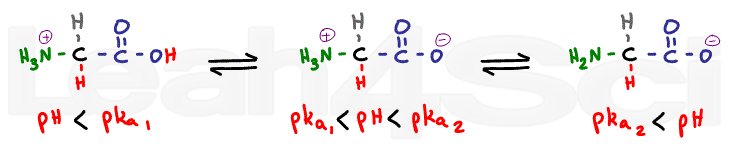
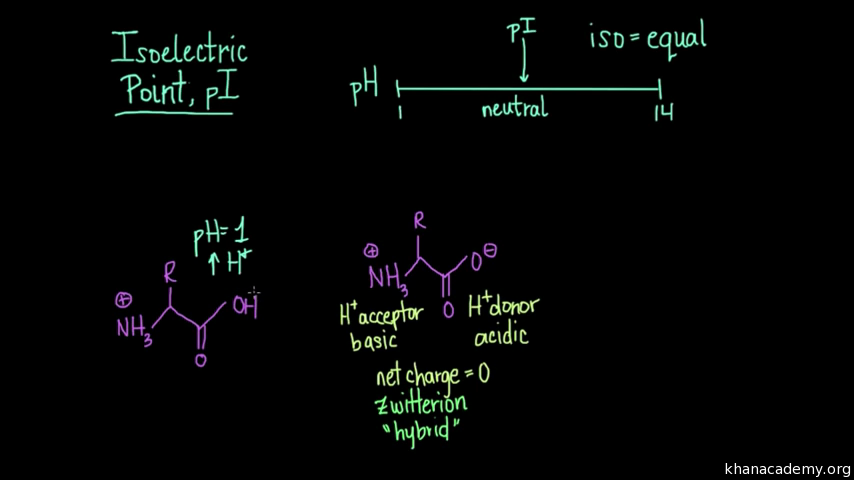
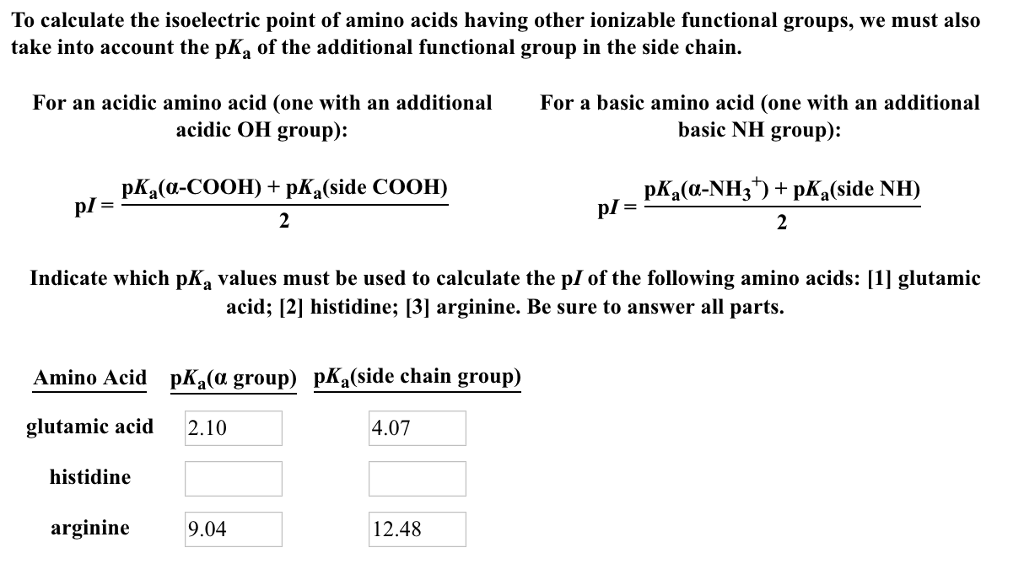
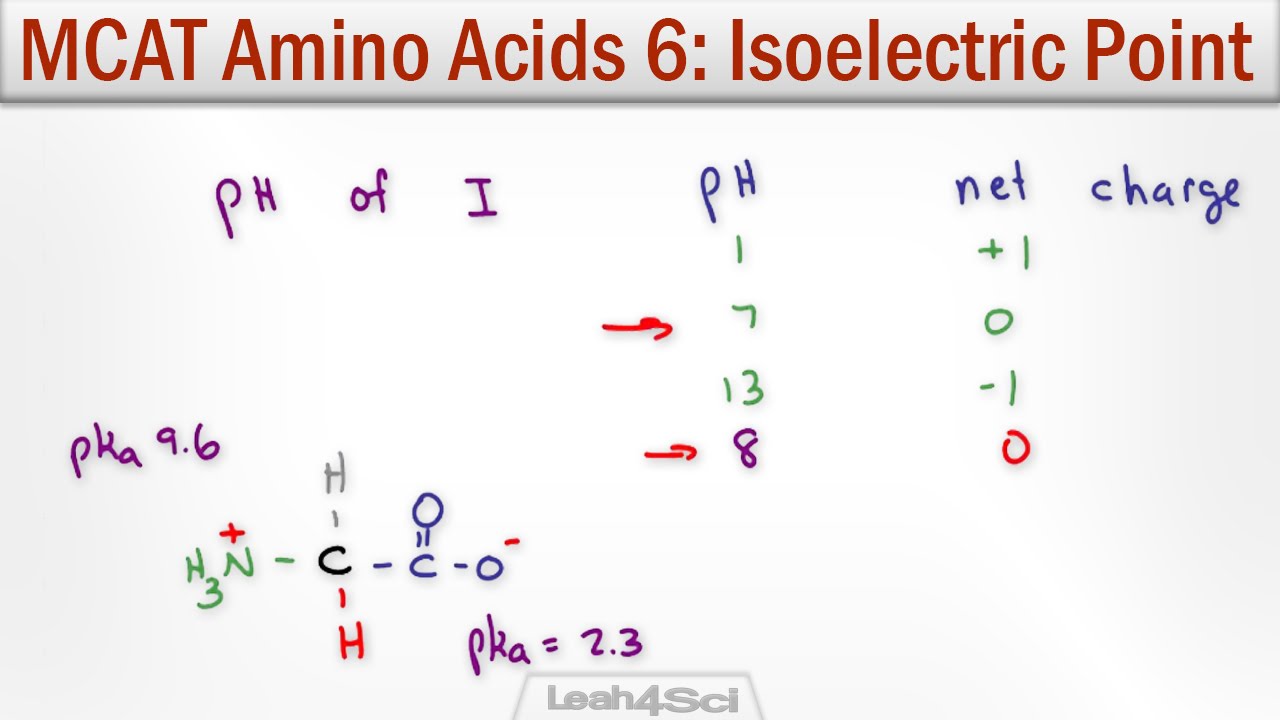
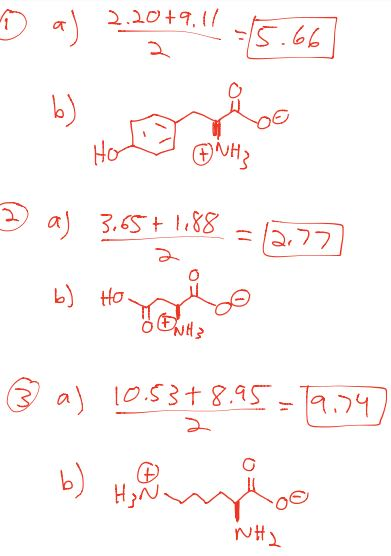
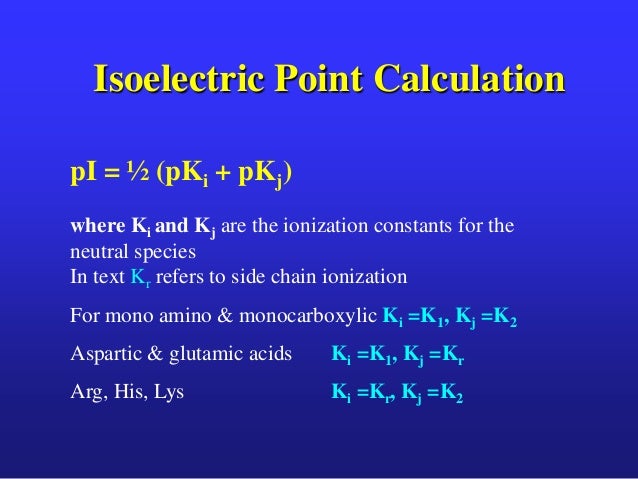
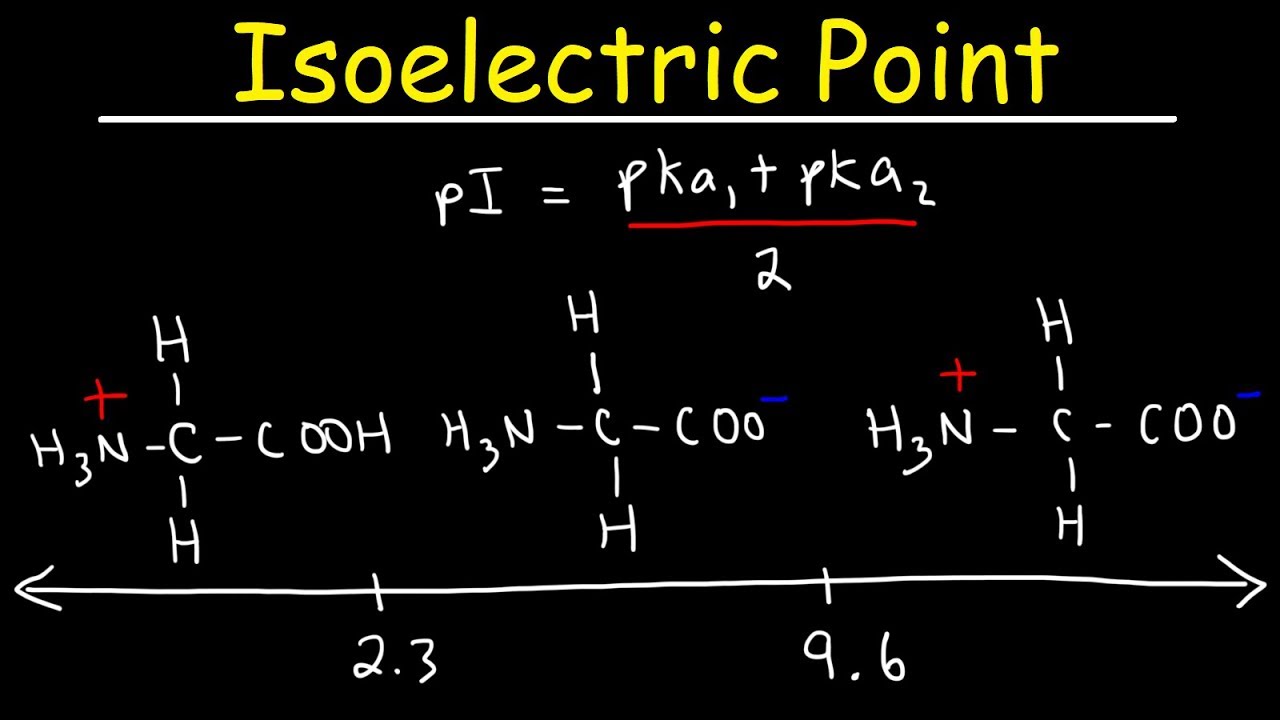
The isoelectric point is the average of the 2 pka values that have a neutral molecule as one of its equilibrium species.

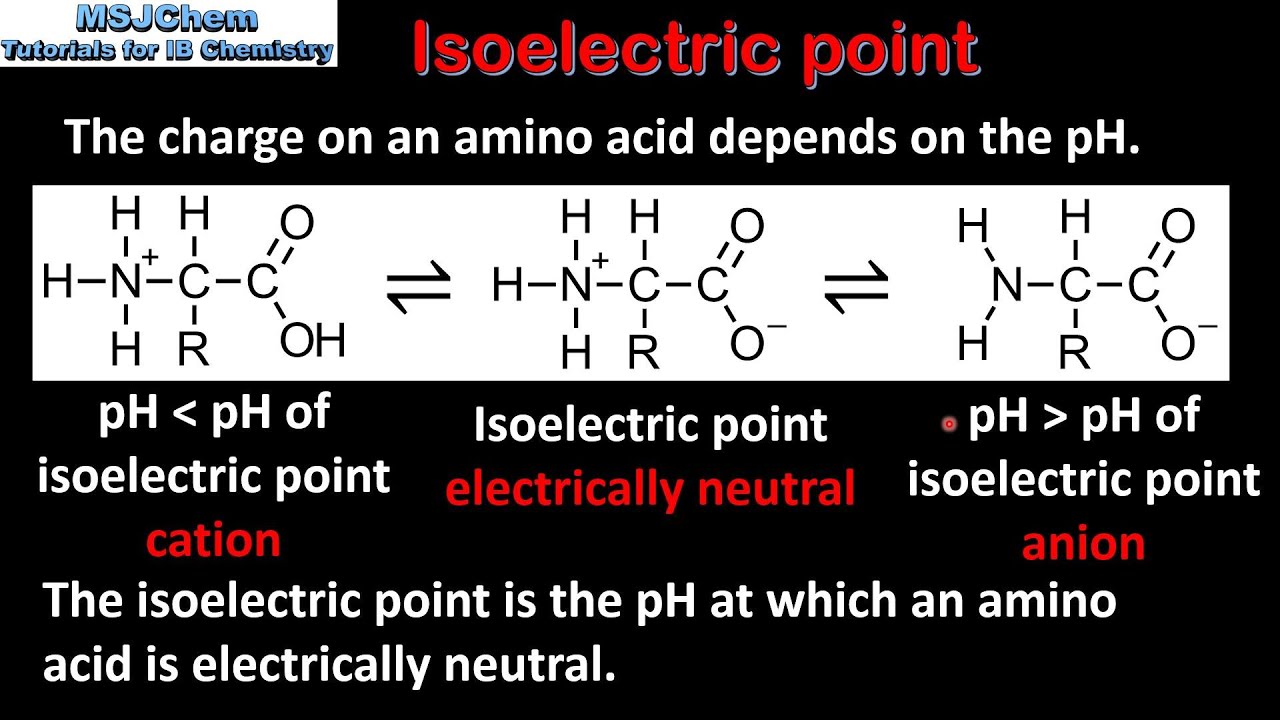
How to calculate isoelectric point. Read the isoelectric point pi value. We will also discuss zwitterions or the forms of amino acids that dominate at the isoelectric point. If the sequence was already available navigate to the expasy server computing tool see resources. When this occurs the isoelectric point pi which is the ph at which the molecule contains no net electric charge can be calculated.
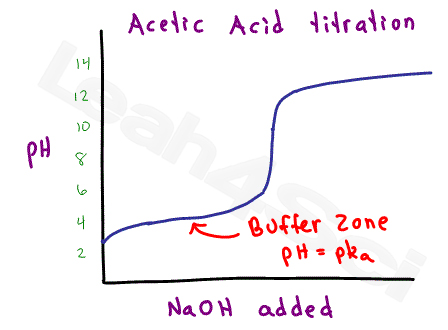
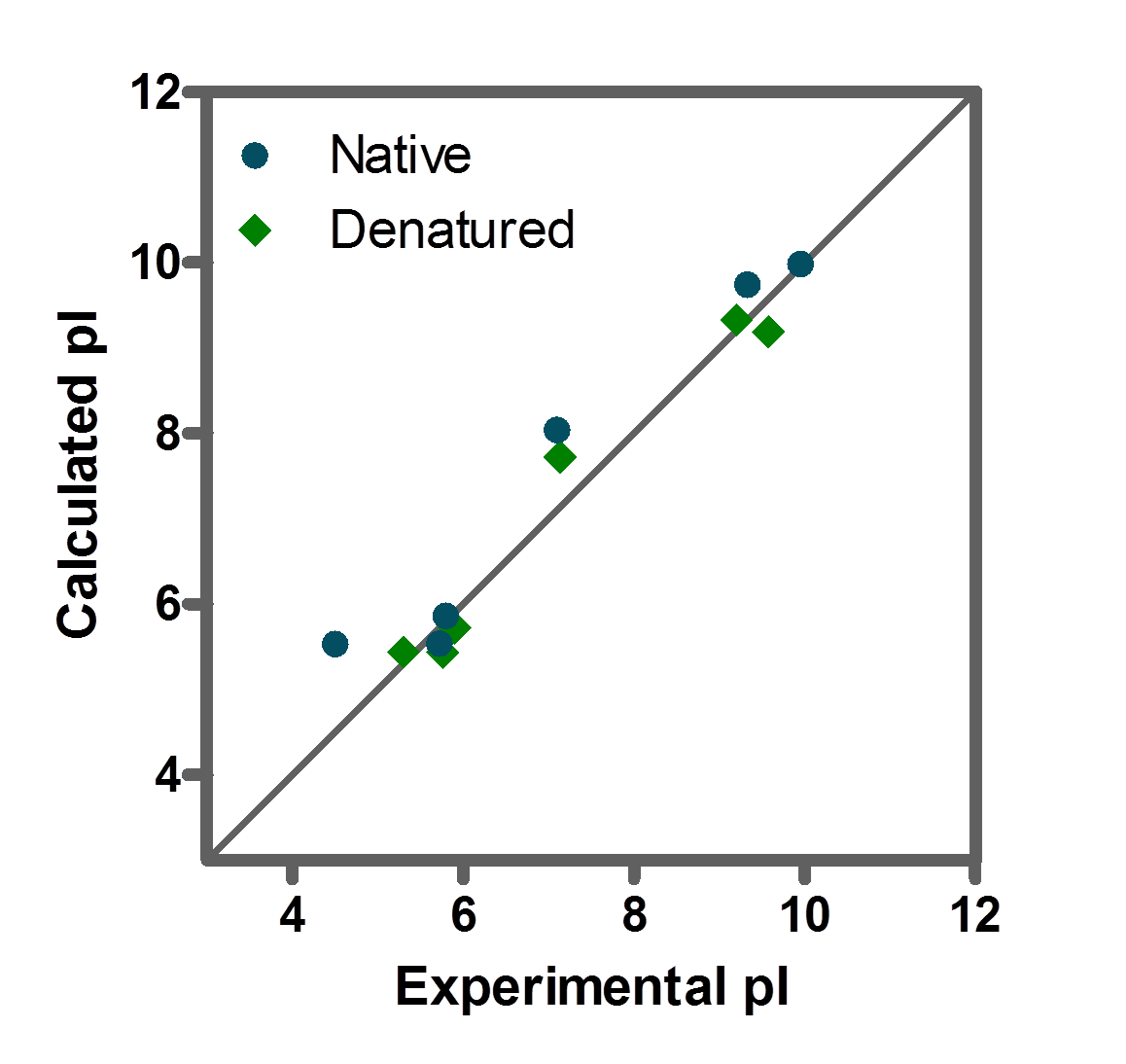
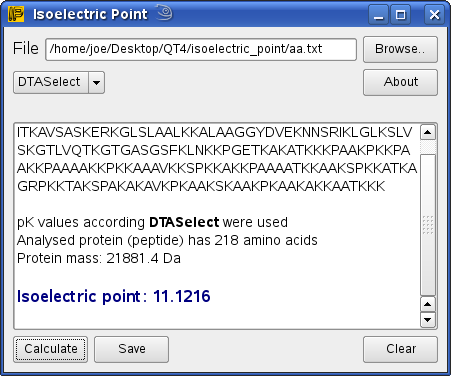
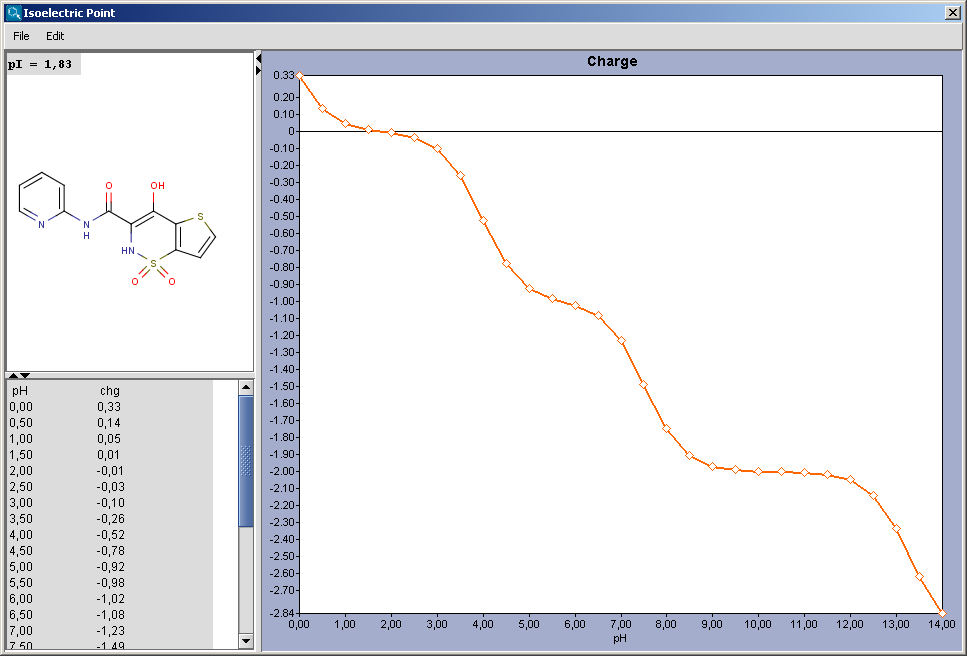
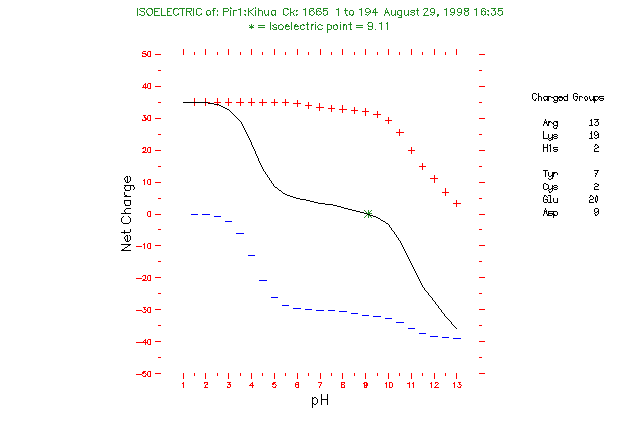
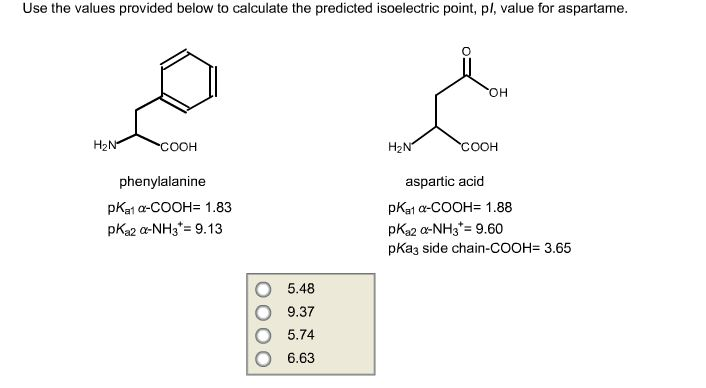
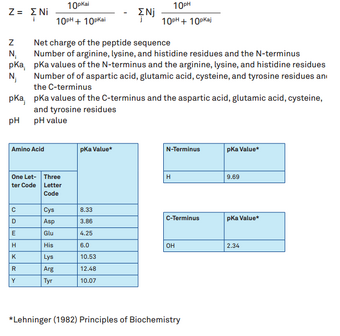
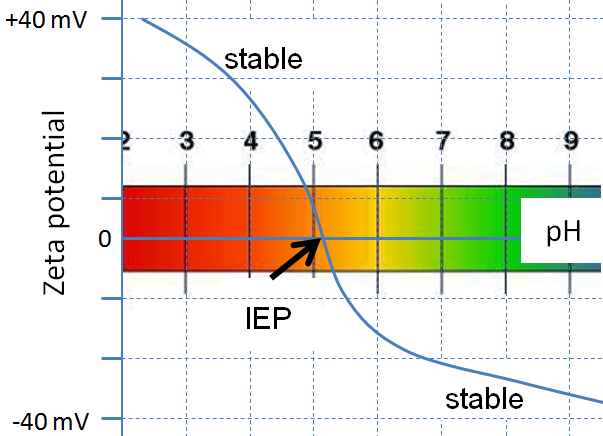
Isoelectric point the ph at which a particular molecule carries no net electrical charge is an critical parameter for many analytical biochemistry and proteomics techniques especially for 2d gel electrophoresis 2d page capillary isoelectric focusing cief x ray crystallography and liquid chromatographymass spectrometry lc ms. Isoelectric point where only the stronger acid group is considered. I 0000235 0000000000339 150 x 10 12 x 189 x 10 14 p1 276 it will be shown in a later publication that the solubility of the undis. Calculation of the isoelectric point from equation 2 gives.
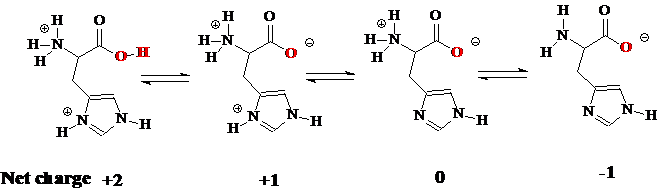
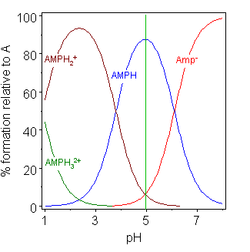
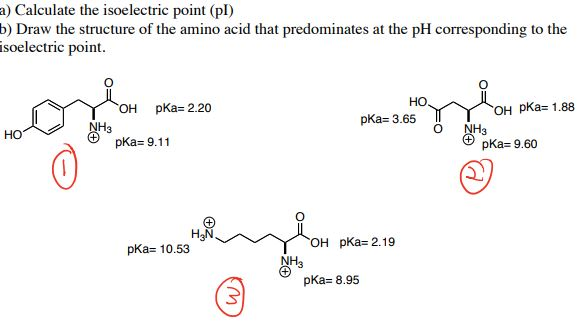
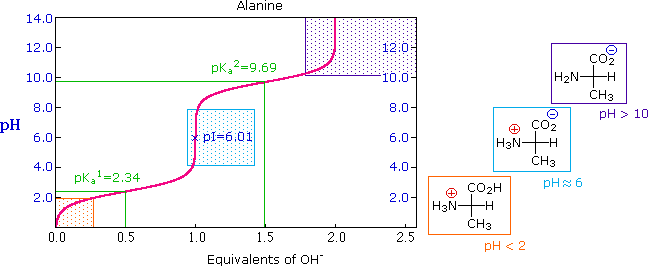
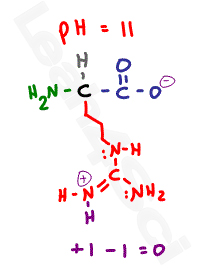
The isoelectric point of an amino acid is the ph at which the amino acid has a neutral charge. The isoelectronic point will be halfway between or the average ofthese two pkas ie. Enter the sequence manually in the field or copy and paste from a file. Isoelectric point of an acidic amino acid aspartic acid isoelectric point pi 21 30 2 30 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 ph 00 05 10 mole fraction h 3n oh o h.
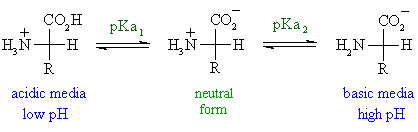
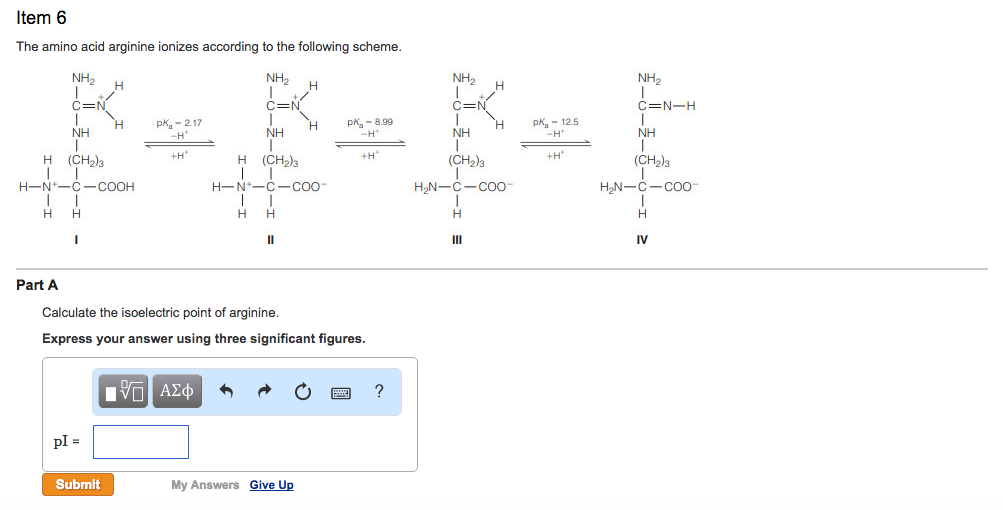
Pi 12 pka1pka2. You will learn how to calculate the isoelectric point and the effects of ph on the amino acids overall charge. Click click here to compute pimw read the isoelectric point pi value. Pka1 and pka2 for thecarboxylic acid and the amine respectively.
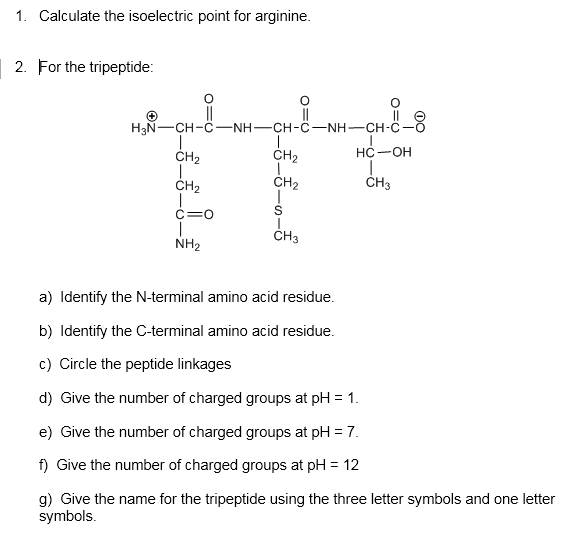
This is the ph of the solution that is required to have the molecule be neutral completely in the zwitterion form. In other words find the pka that takes the amino acid from neutral to 1 960 for glycine find the pka value that takes the amino acid from neutral to 1 234 for glycine and then find the halfway point or average. Enter the peptide one letter sequenceaselp in our examplein the box and click on compute read the isoelectric point pi value given in the line theoretical pimw in our example the pi is 400.

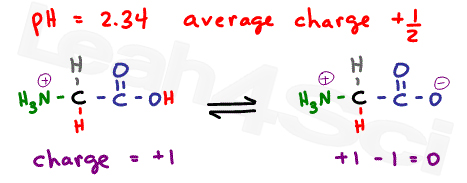


.jpg&ts=20111101023600&ri=200)













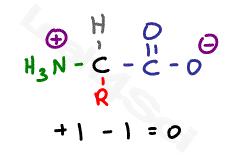




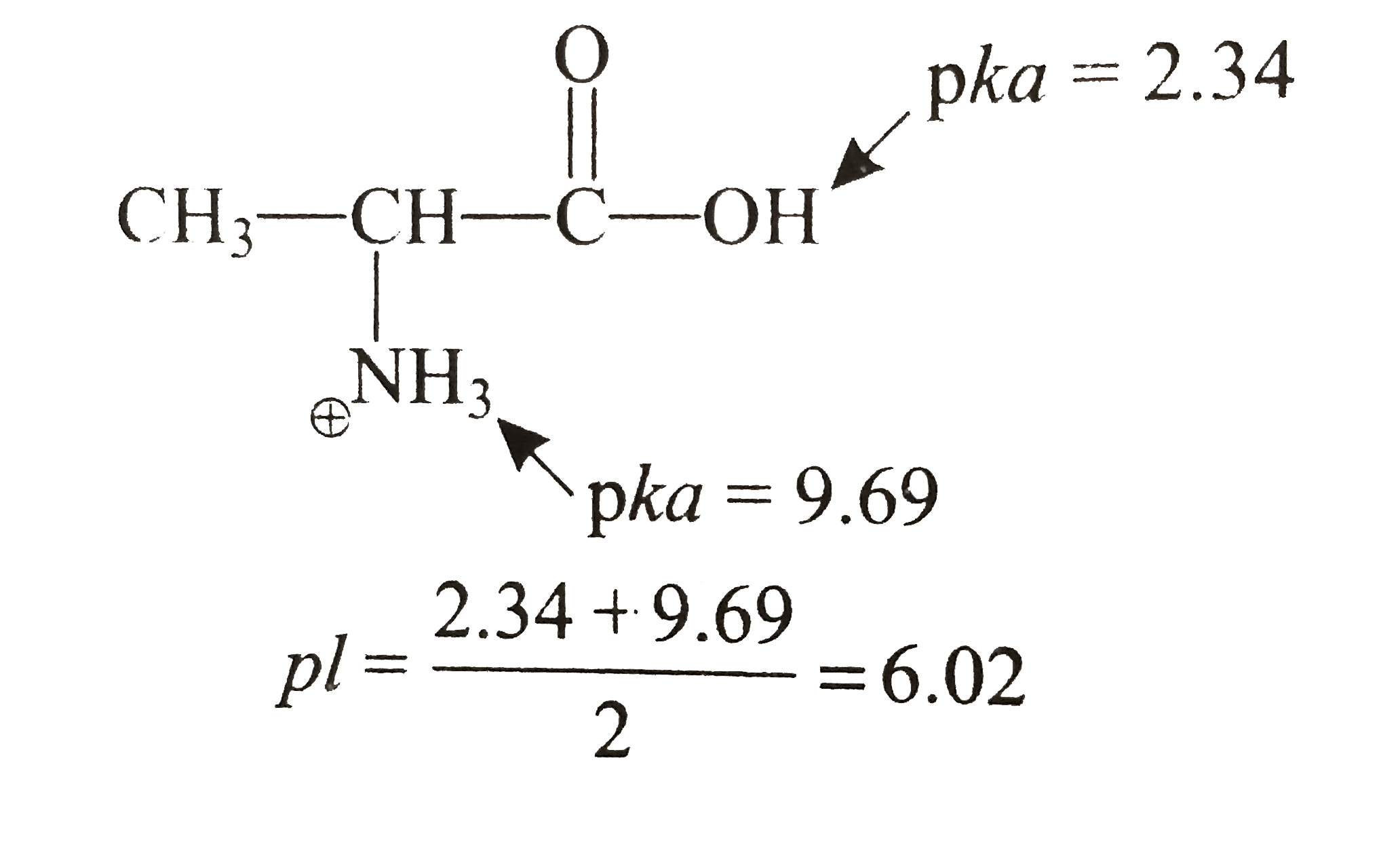








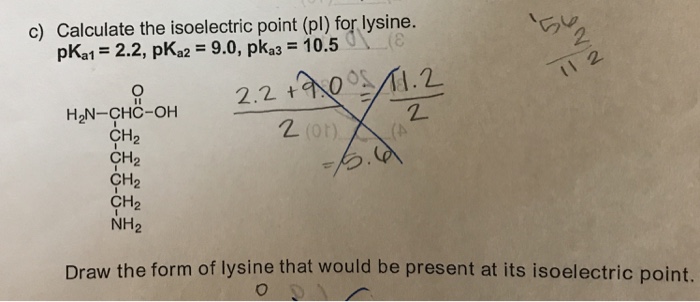















.jpg)