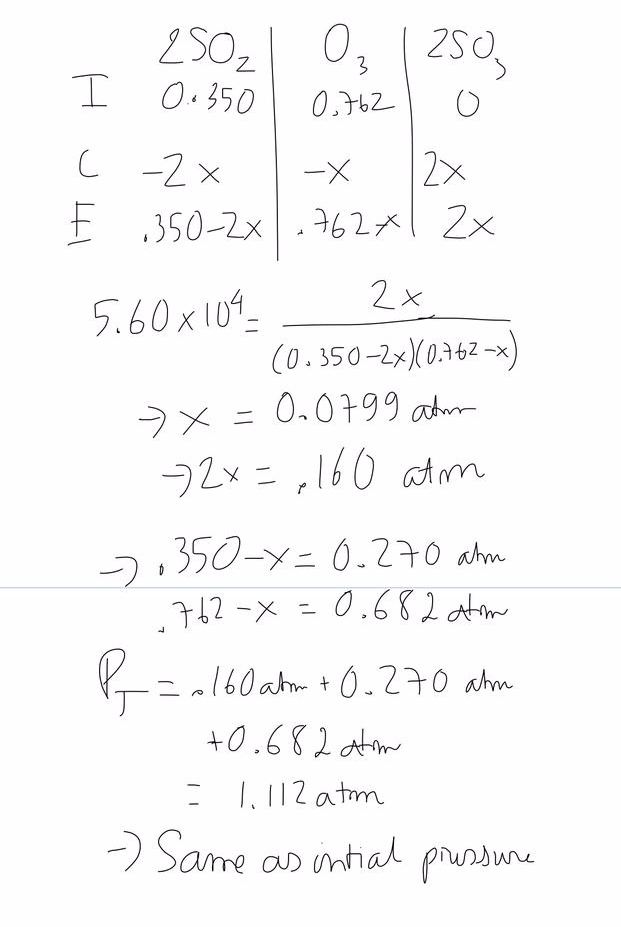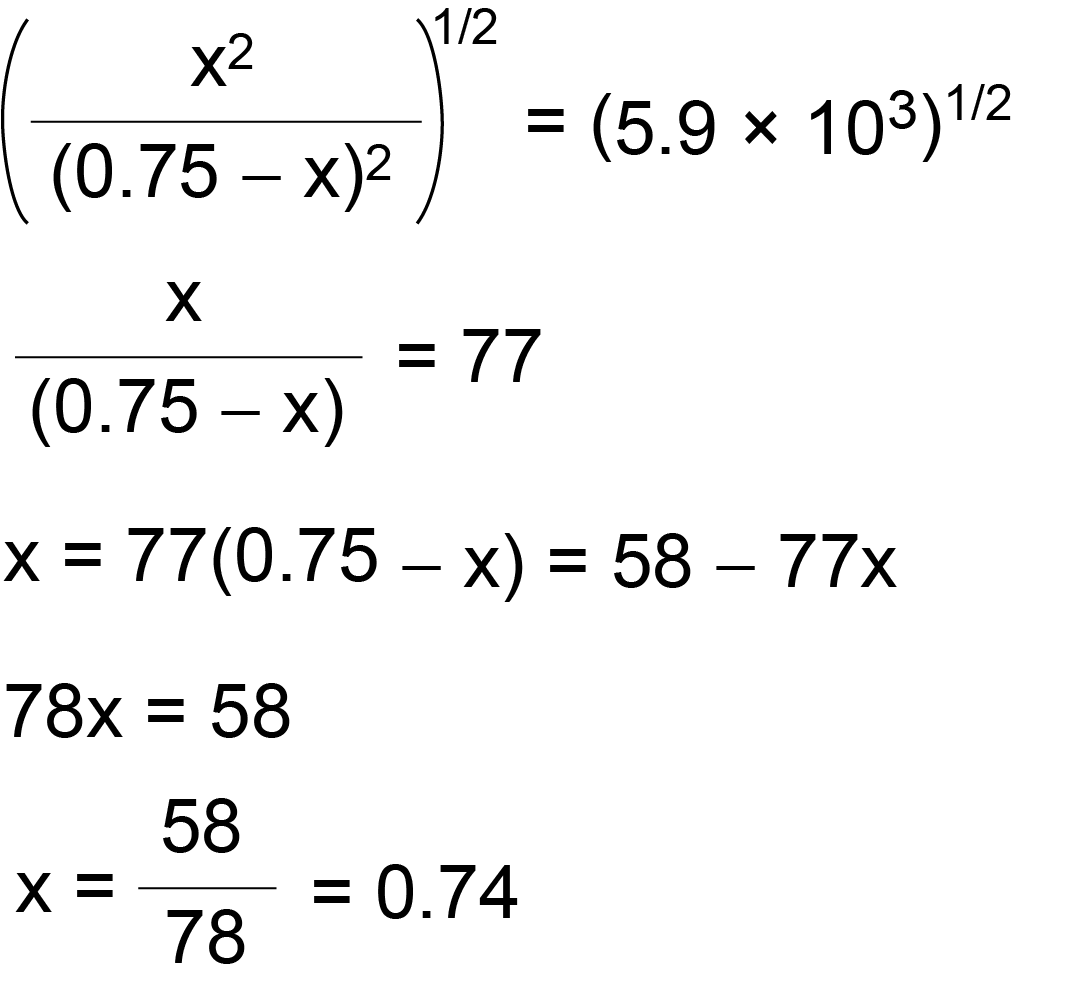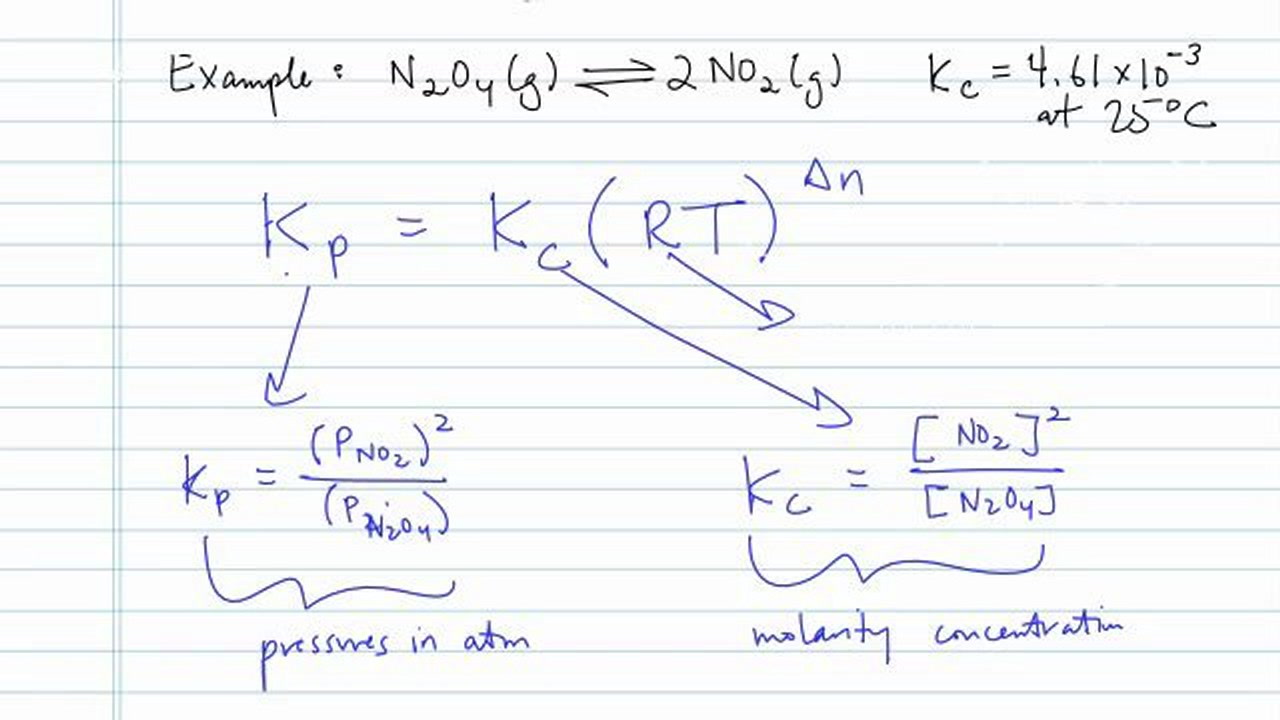How To Calculate Equilibrium Constant Kp
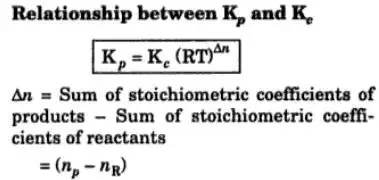
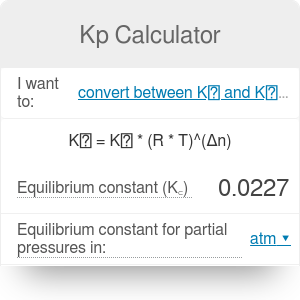
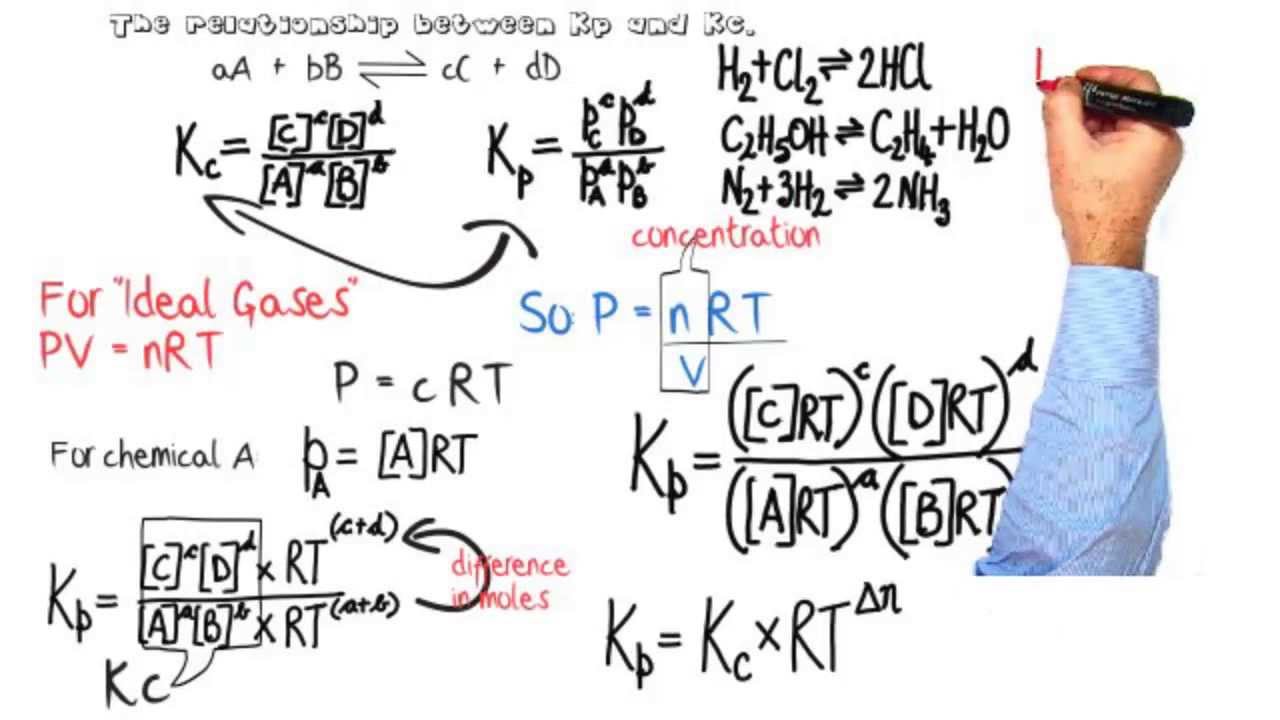
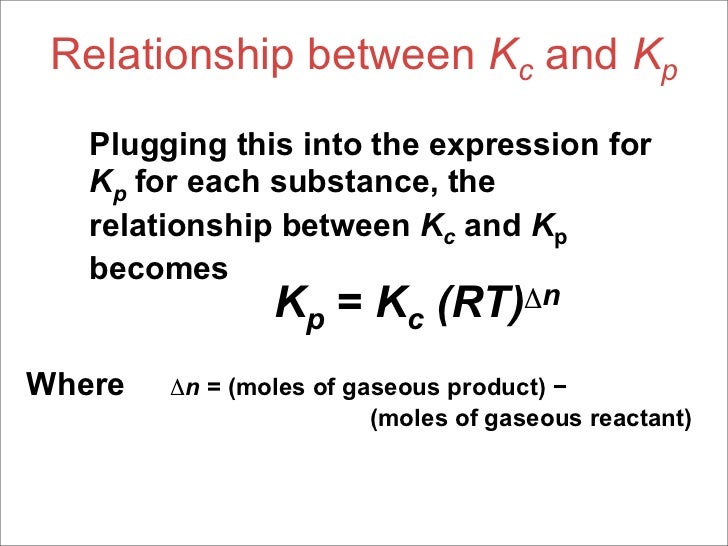
Because we do not choose to use units for k c and k p we cannot cancel units for r and t.
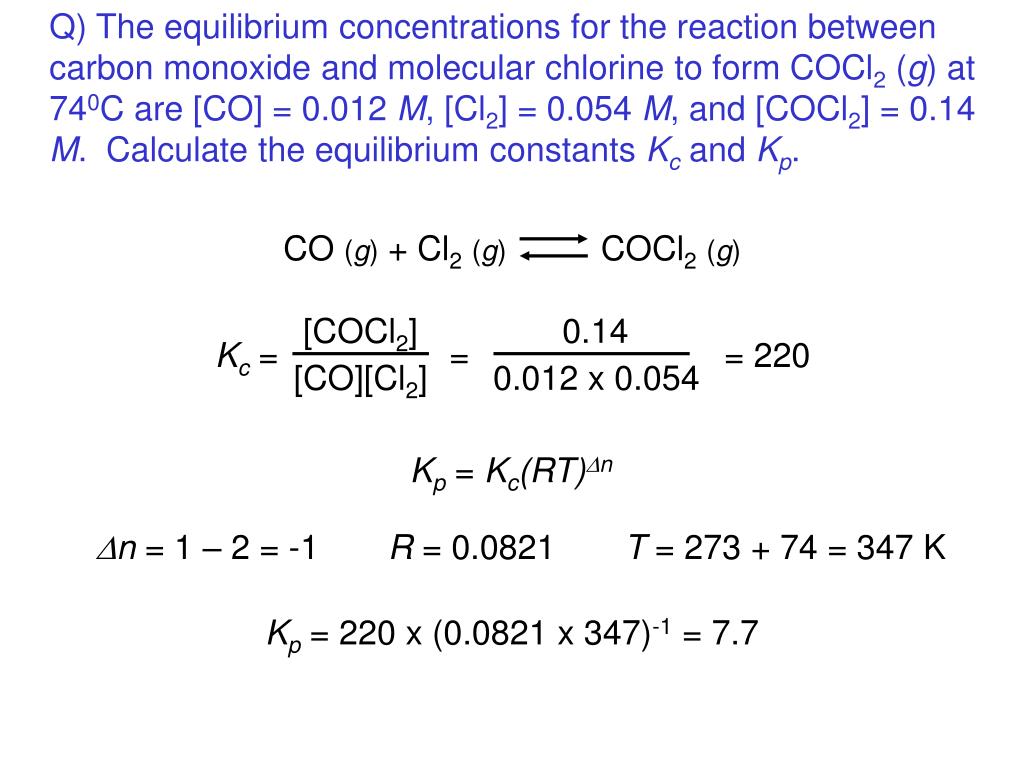
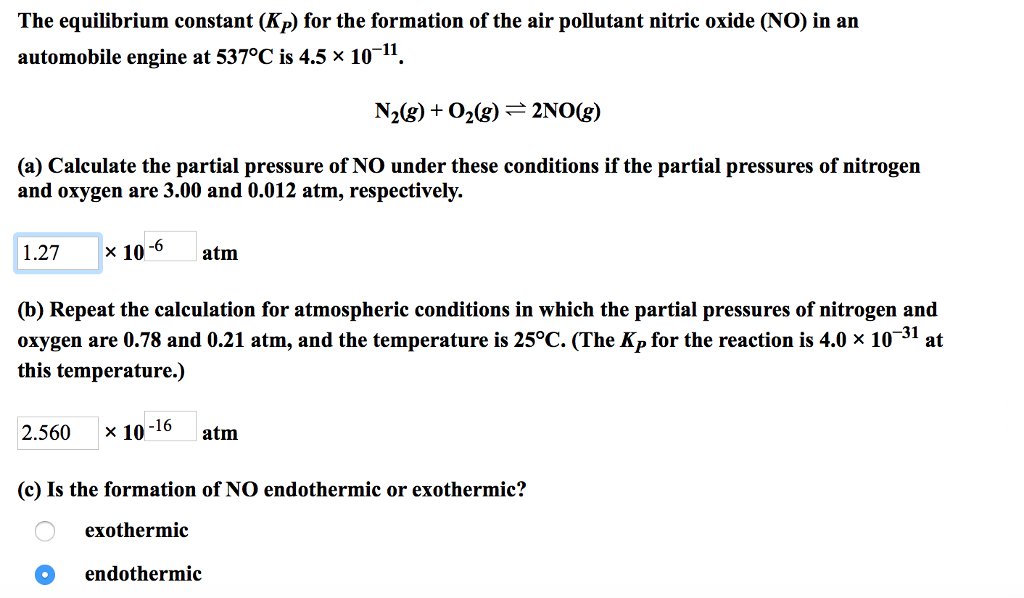
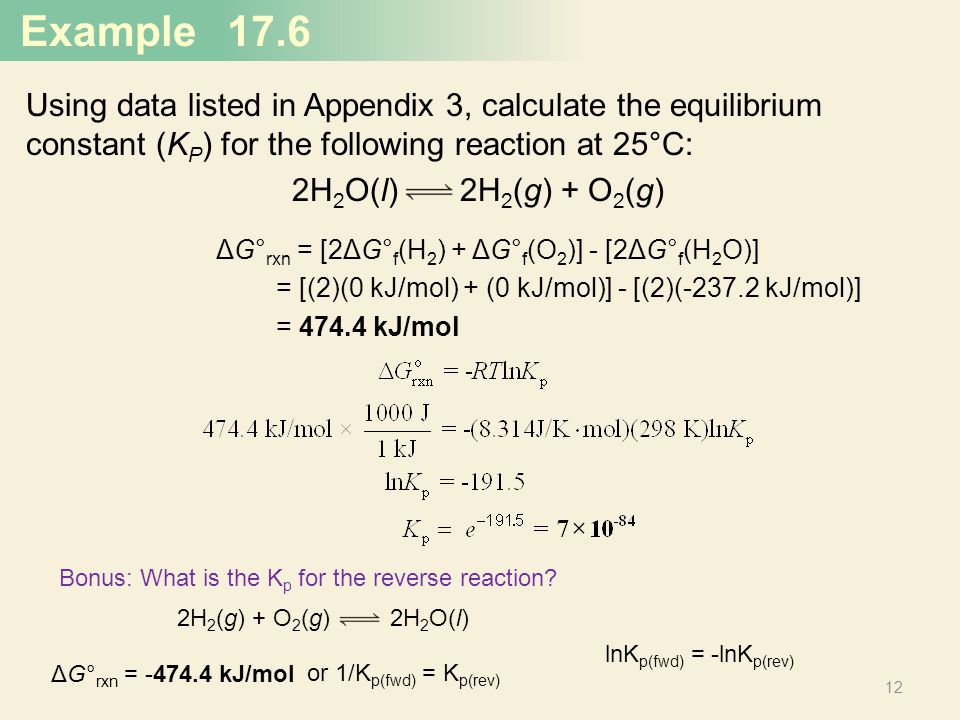
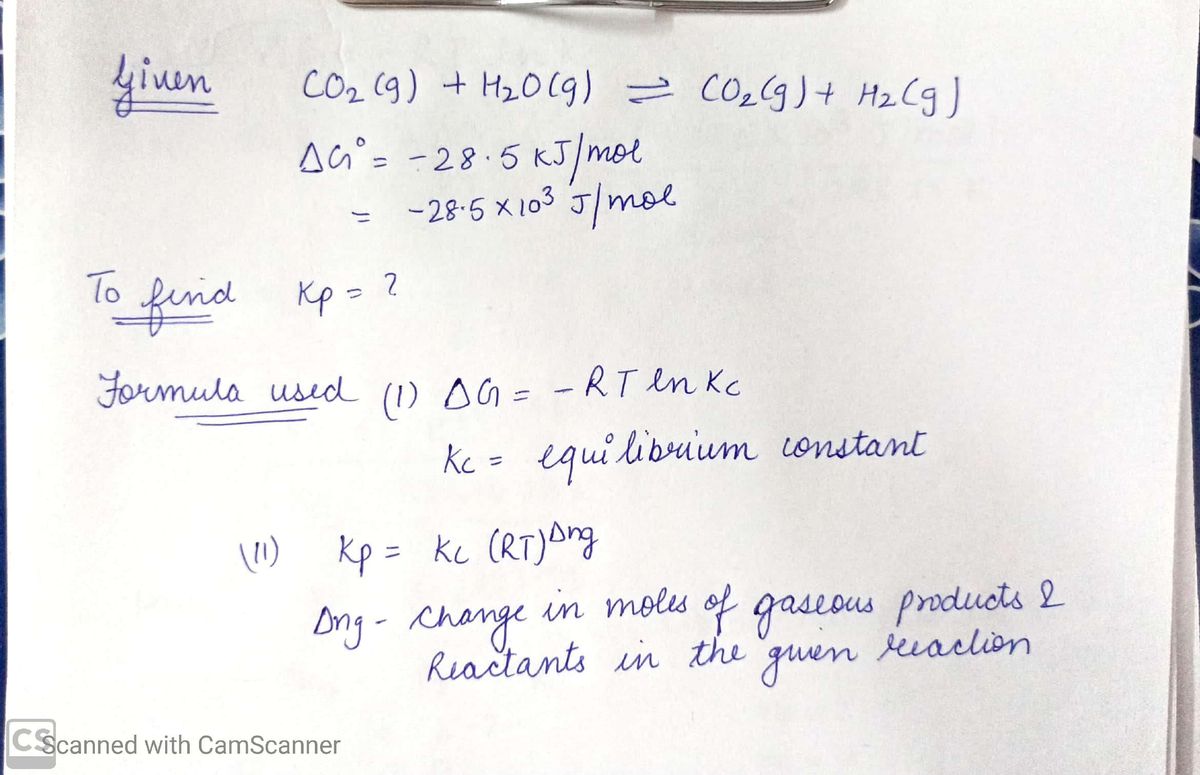
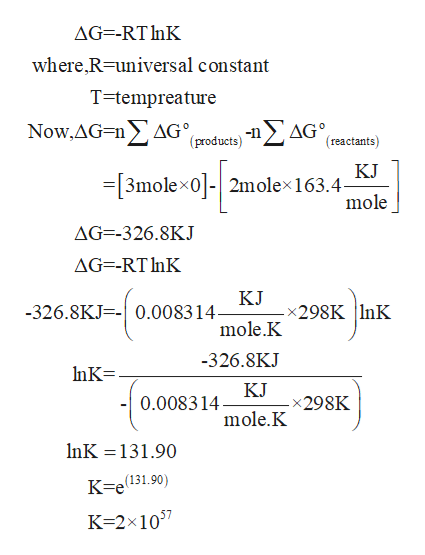
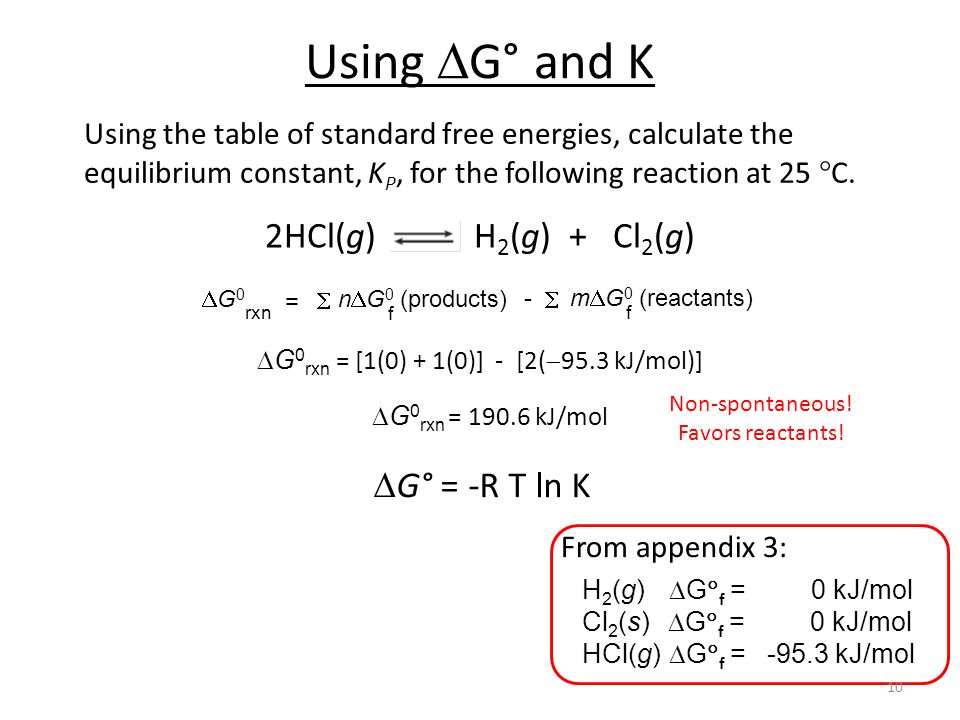
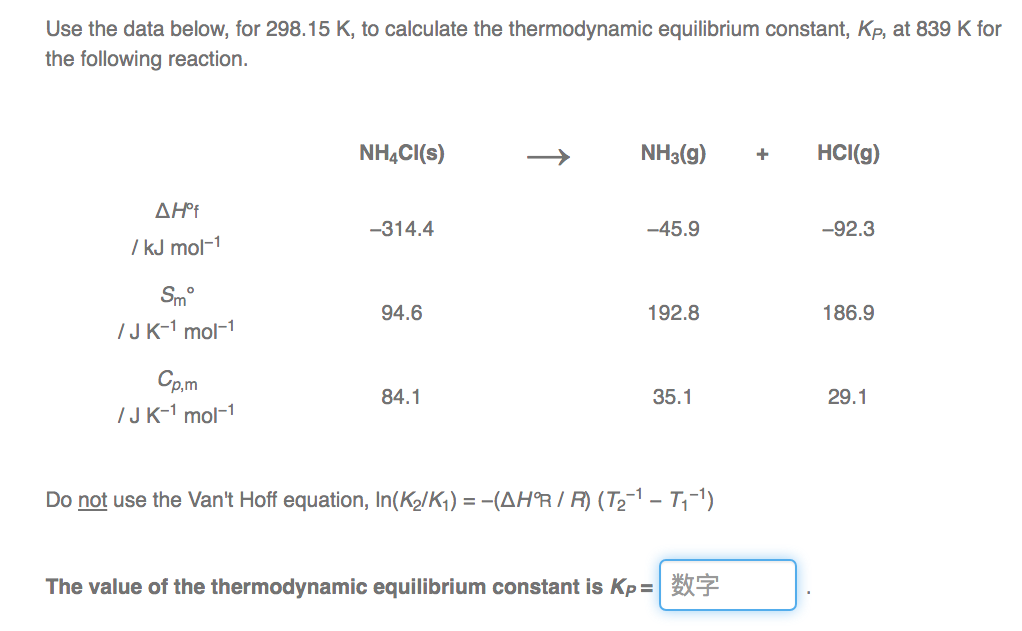
How to calculate equilibrium constant kp. Calculating an equilibrium constant from the free energy change if we know the standard state free energy change go for a chemical process at some temperature t we can calculate the equilibrium constant for the process at that temperature using the relationship between goand k. Dn 2 moles of gaseous products 0 moles of gaseous reactants 2. Use the coefficients in the balanced chemical. Give the equilibrium constant expression for the generation of water from oxygen and hydrogen gas.
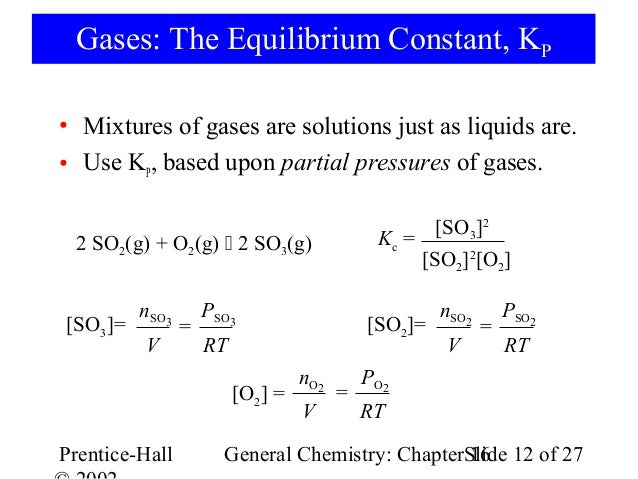
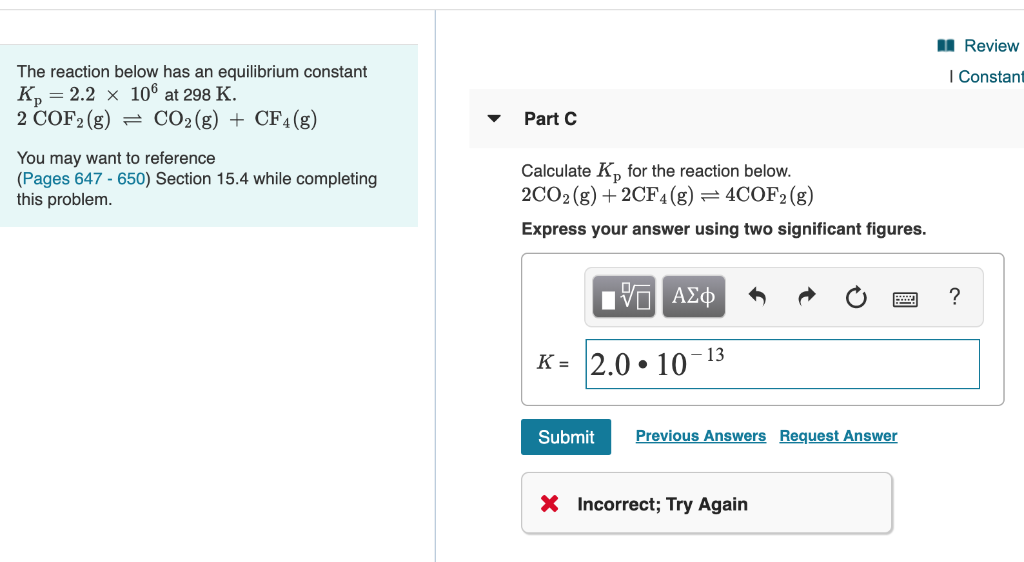
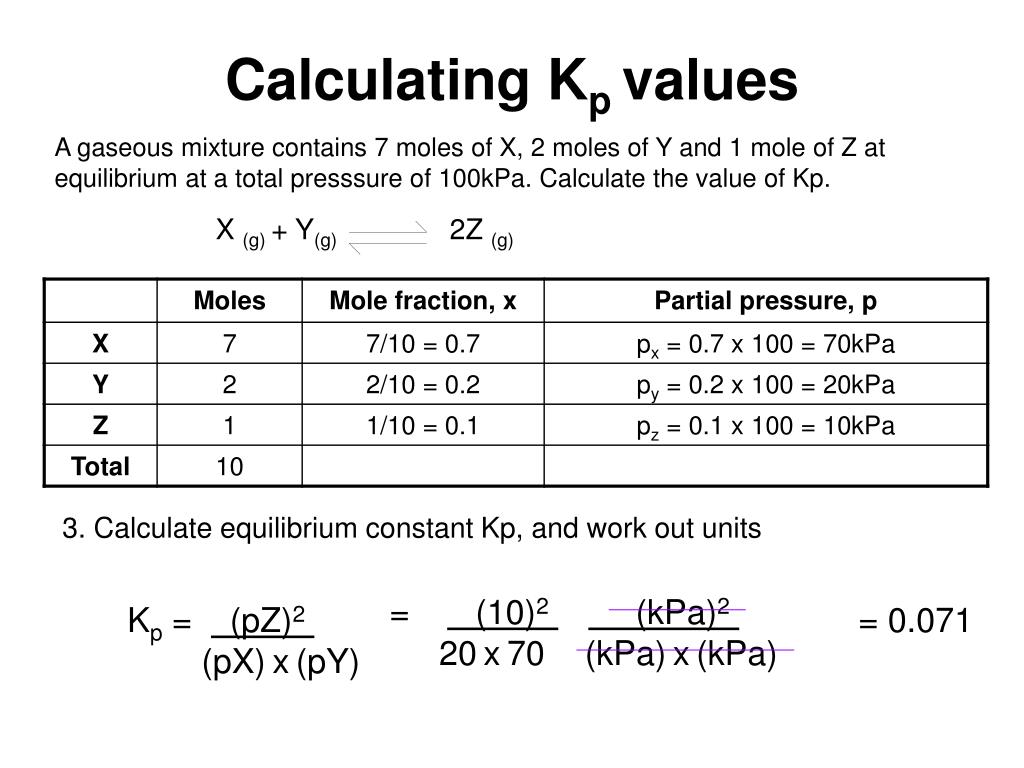
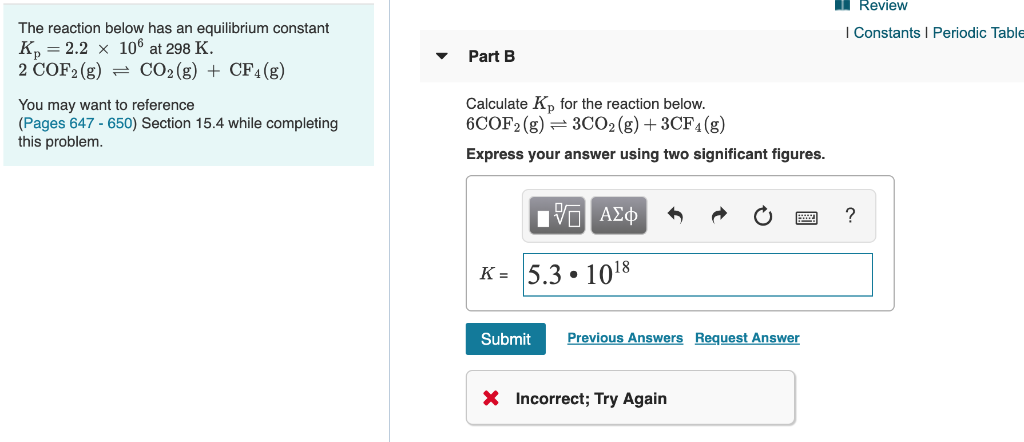
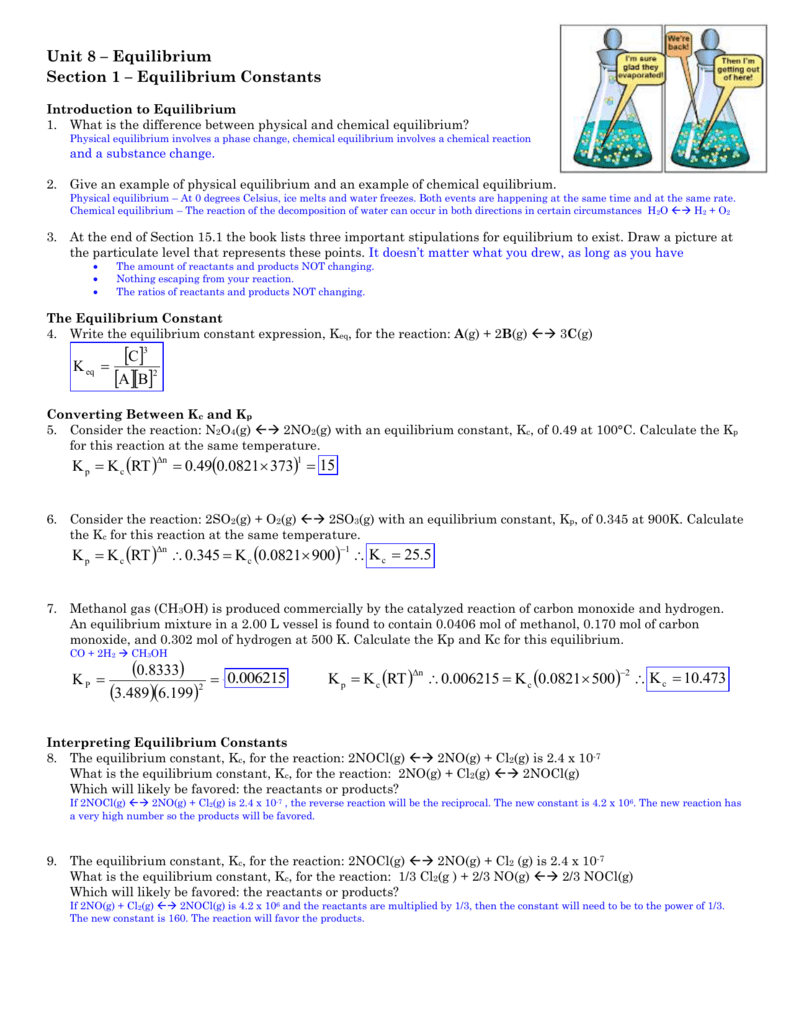
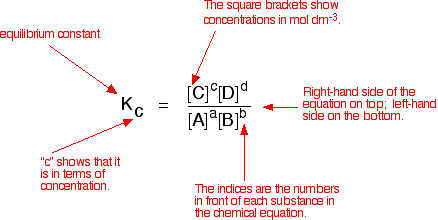
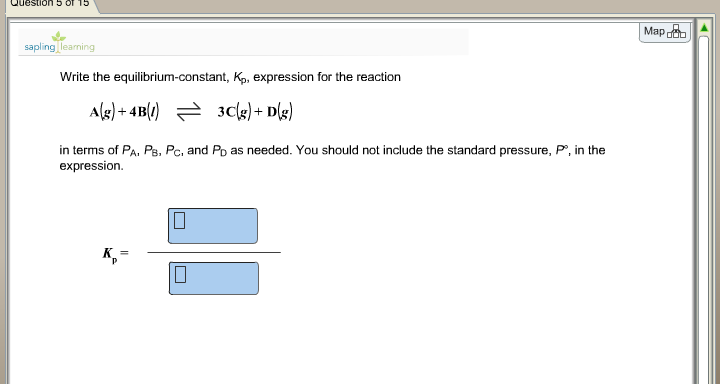
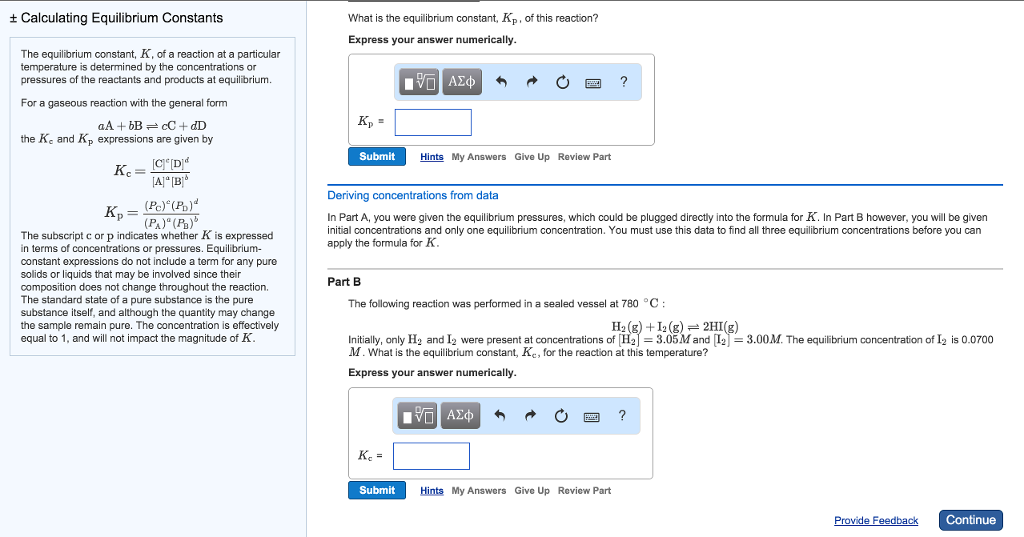
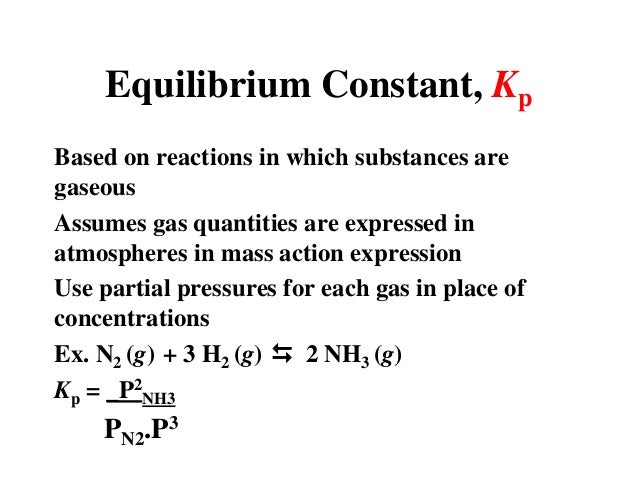
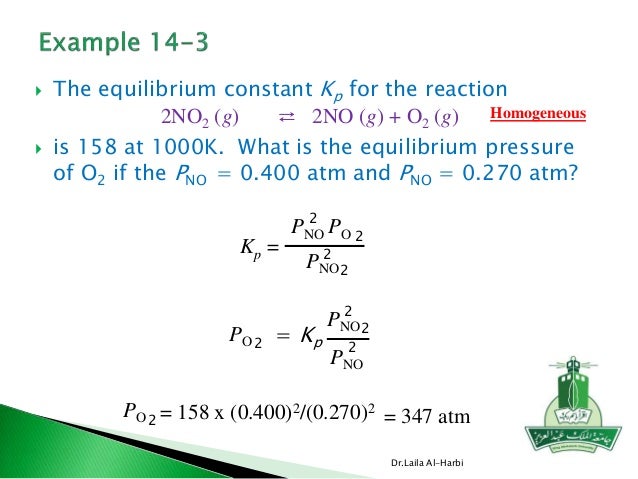
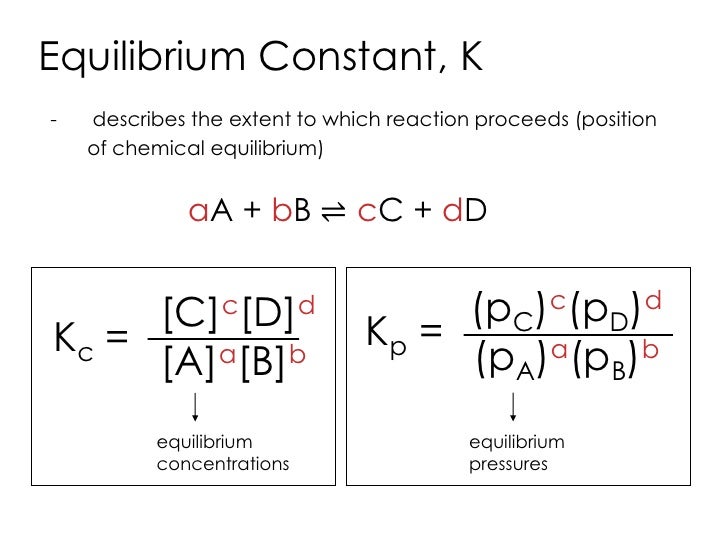
K p 696 x 10 500821333 2 0052. We are going to start by looking at a general case with the equation. When the equilibrium constant is written with the gases in terms of partial pressure the equilibrium constant is written as the symbol ktext p k p. Kc ccdd aabb.
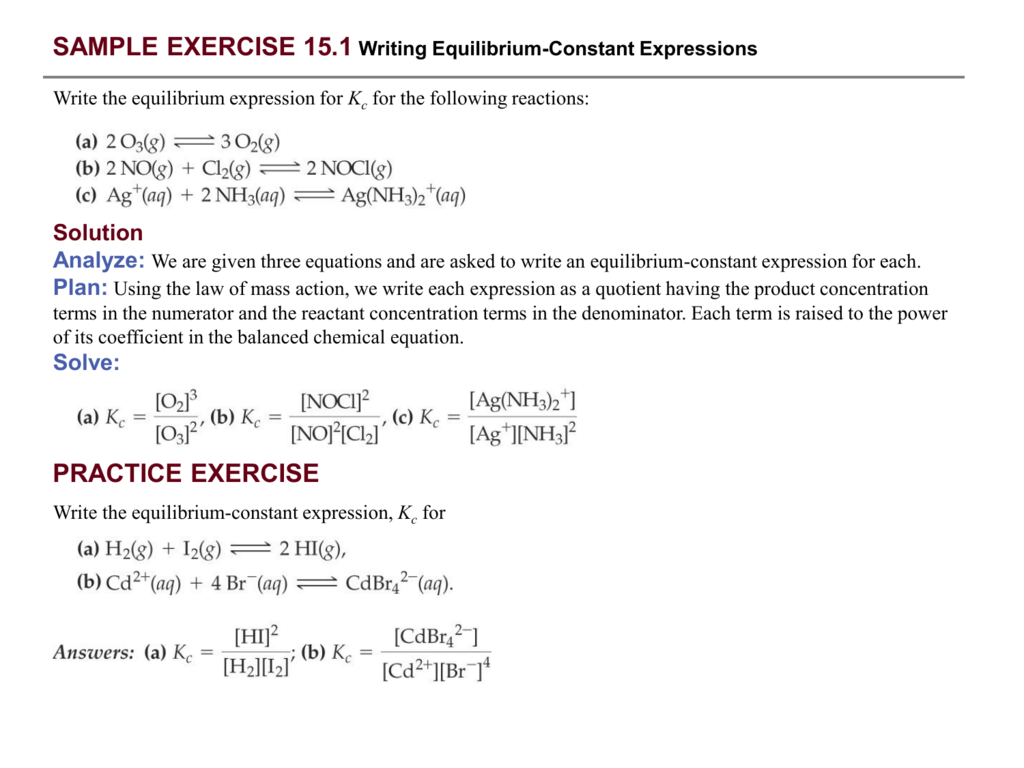
In both cases the equations are fairly similar. Construct a table showing the initial concentrations the changes in concentrations. 2h2g o2g rightleftharpoons 2h2og 2. Write the equilibrium constant expression for the reaction.
Writing an expression for k p. Reversible reactions equilibrium and the equilibrium constant k. 2so2 o2 rightleftharpoons 2so3 3. Calculate all possible initial concentrations from the data given and insert them in the table.
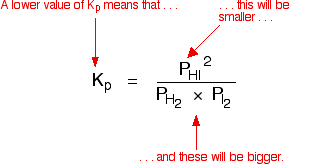
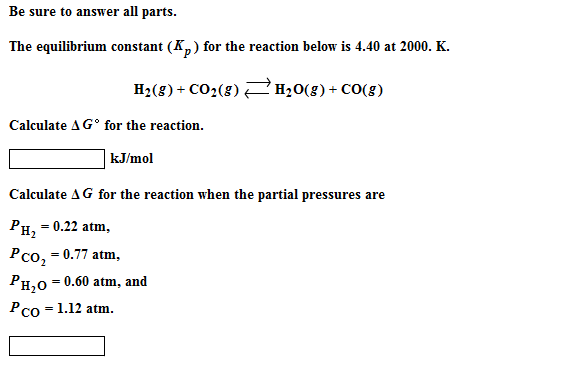
How to calculate k and how to use k to determine if a reaction strongly favors products or reactants at equilibrium. See solubility product constant k sp the standard example of writing gas equilibrium constants are. If any of the reactants or products are solids or liquids their concentrations are equal to one because they are pure substances. If both the forward and backward reactions occur simultaneously then it is known as a reversible reaction.
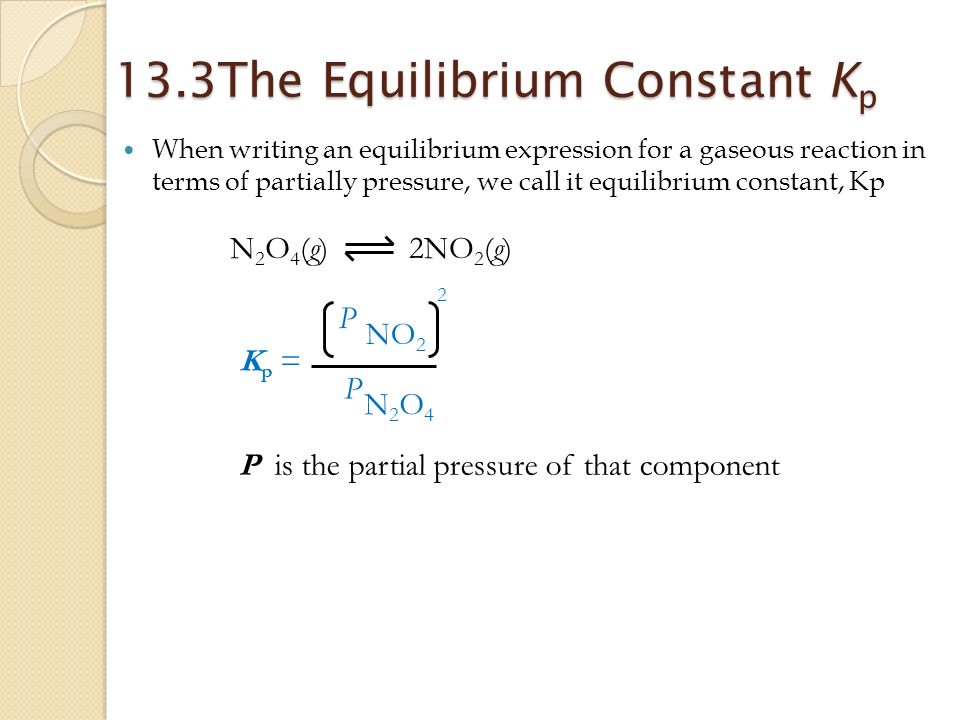
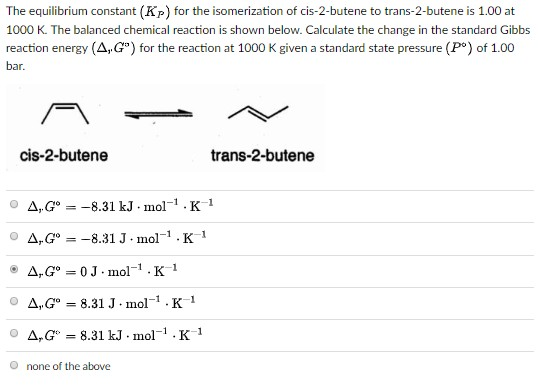
What is kp given that the initial pressure of so3 is 3 atm and zero for the other species for this reversible reaction. In such cases you can calculate the equilibrium constant by using the molar concentrationkc of the chemicals or by using their partial pressurekp. Kp ccdd aabb. When a reaction component is a gas we can also express the amount of that chemical at equilibrium in terms of its partial pressure.
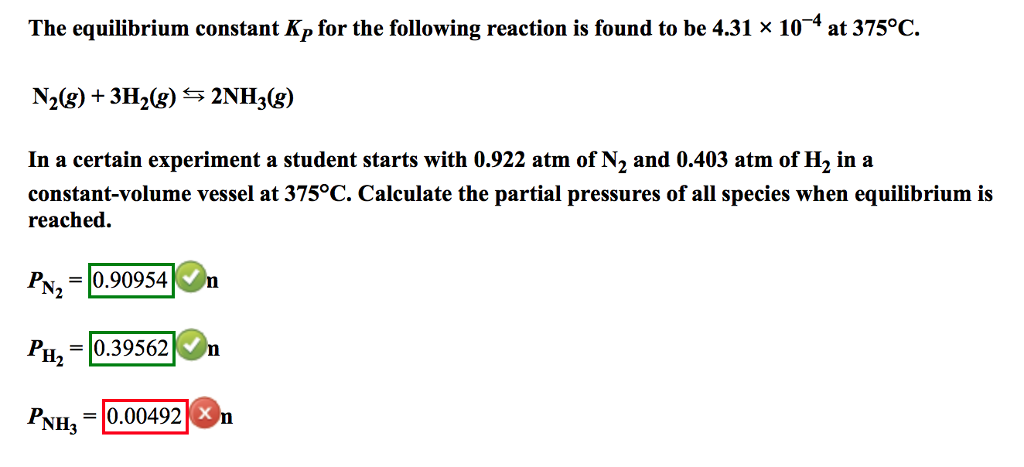
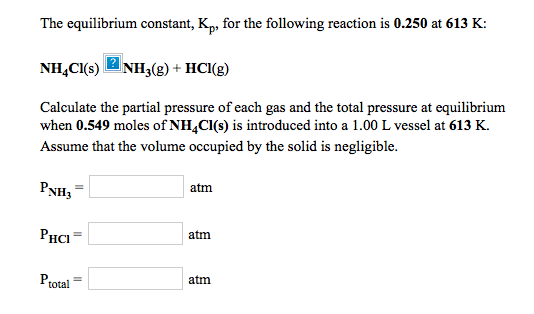
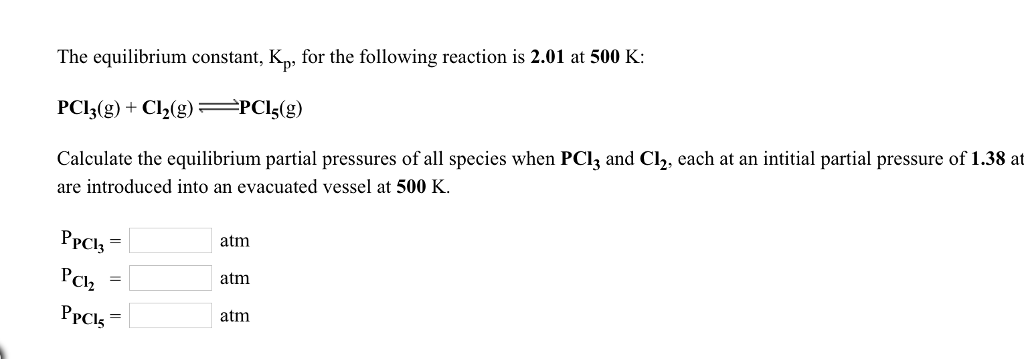
Create an ice chartand calculate the changesin pressure and equilibrium pressures for each species. Calculate the difference in the number of moles of gases dn. Check to see that the given amounts are measured in appropriate pressure units since kpis to be.