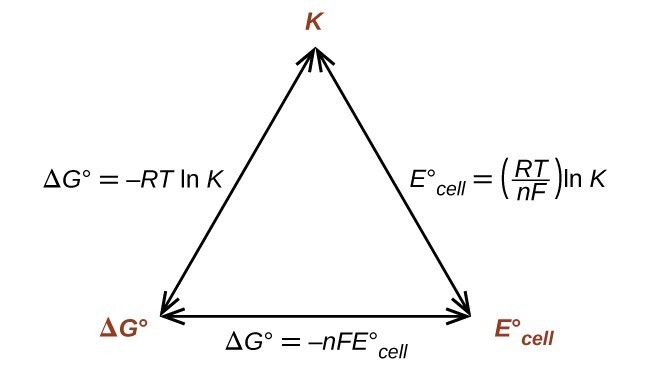
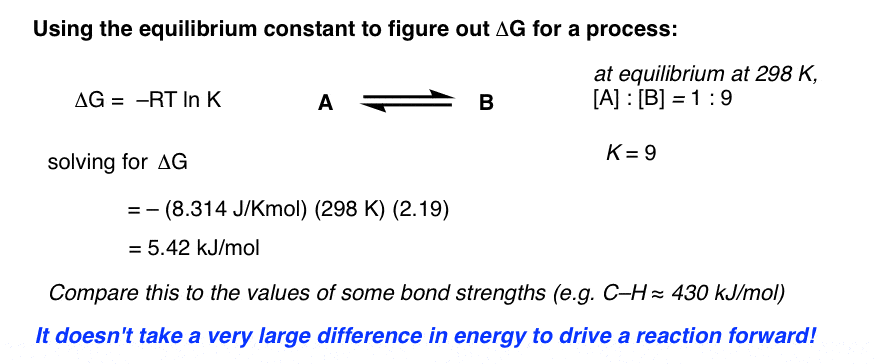
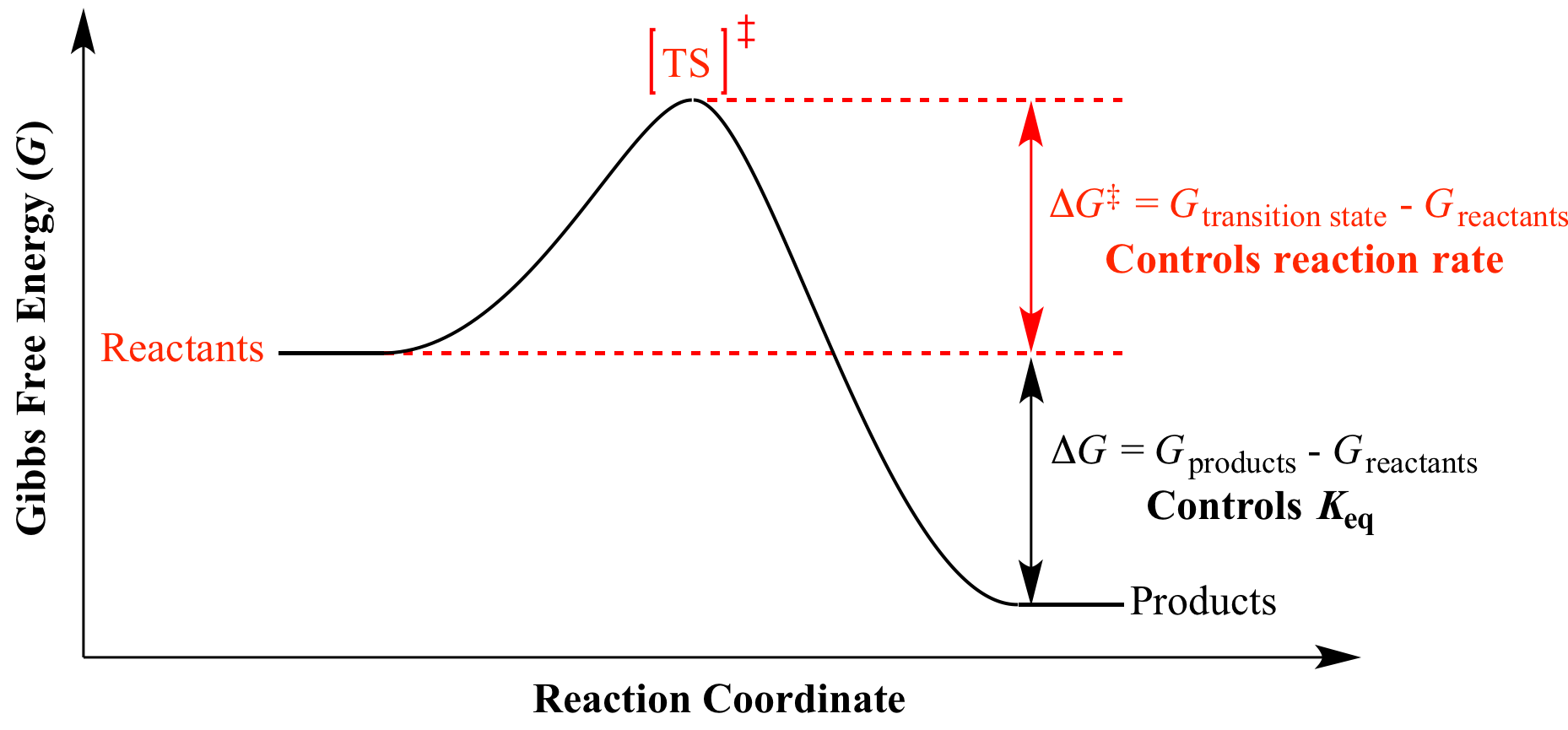
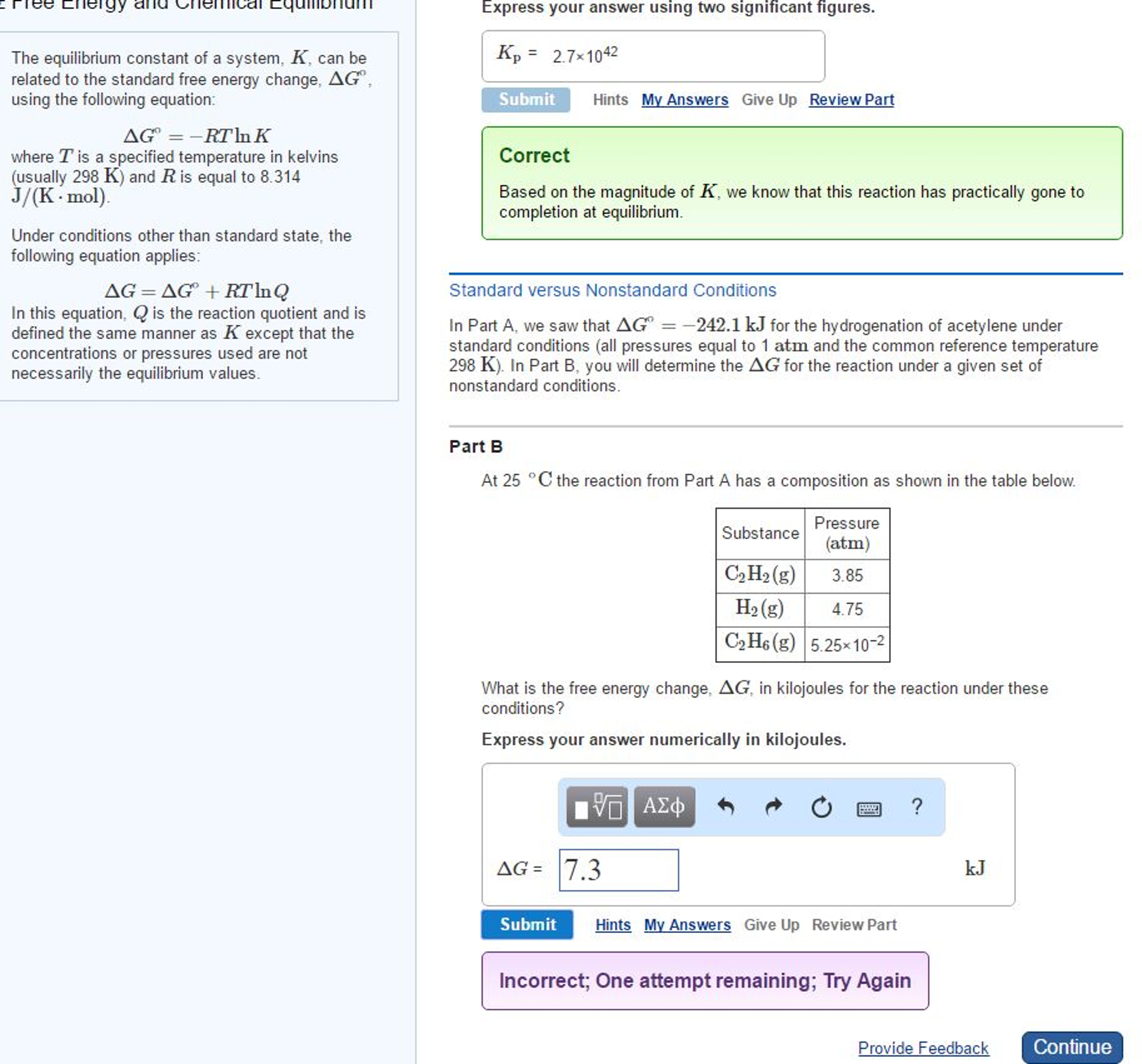
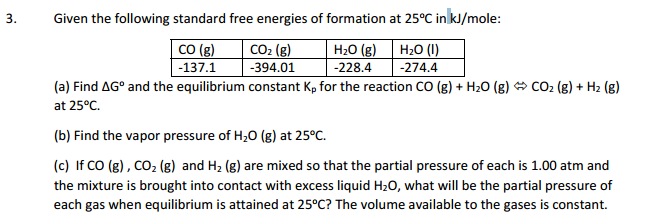
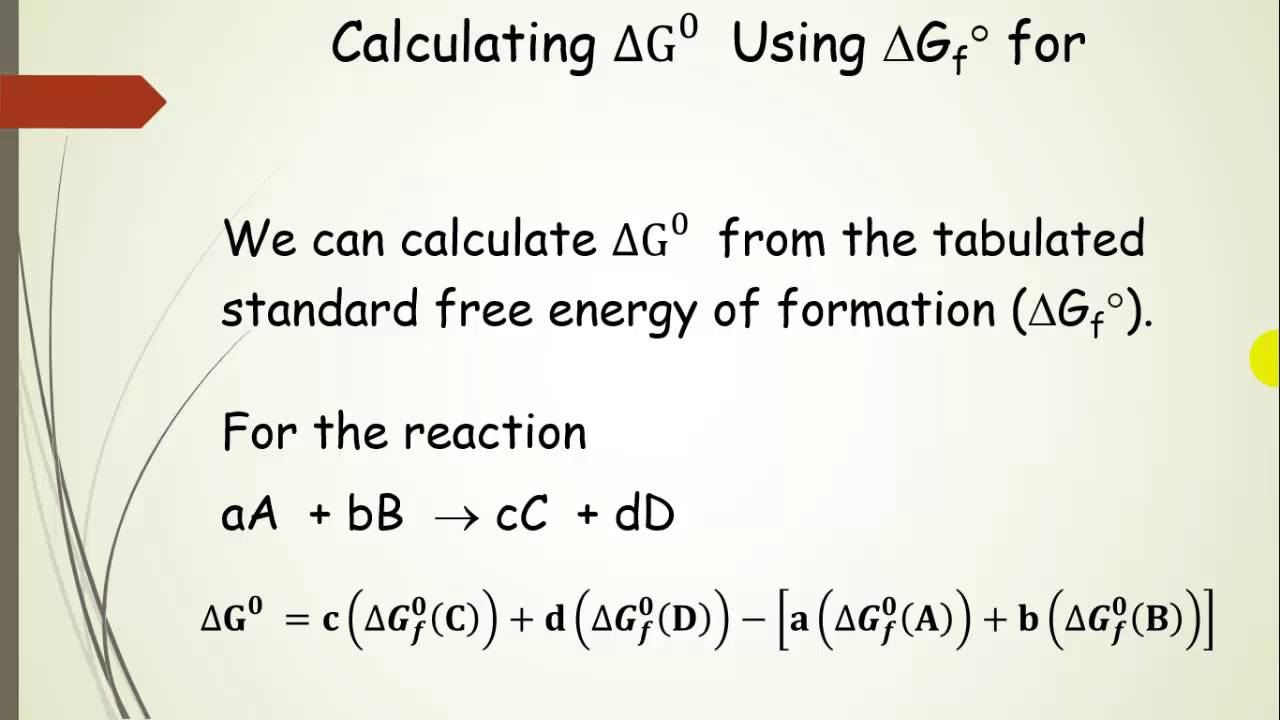
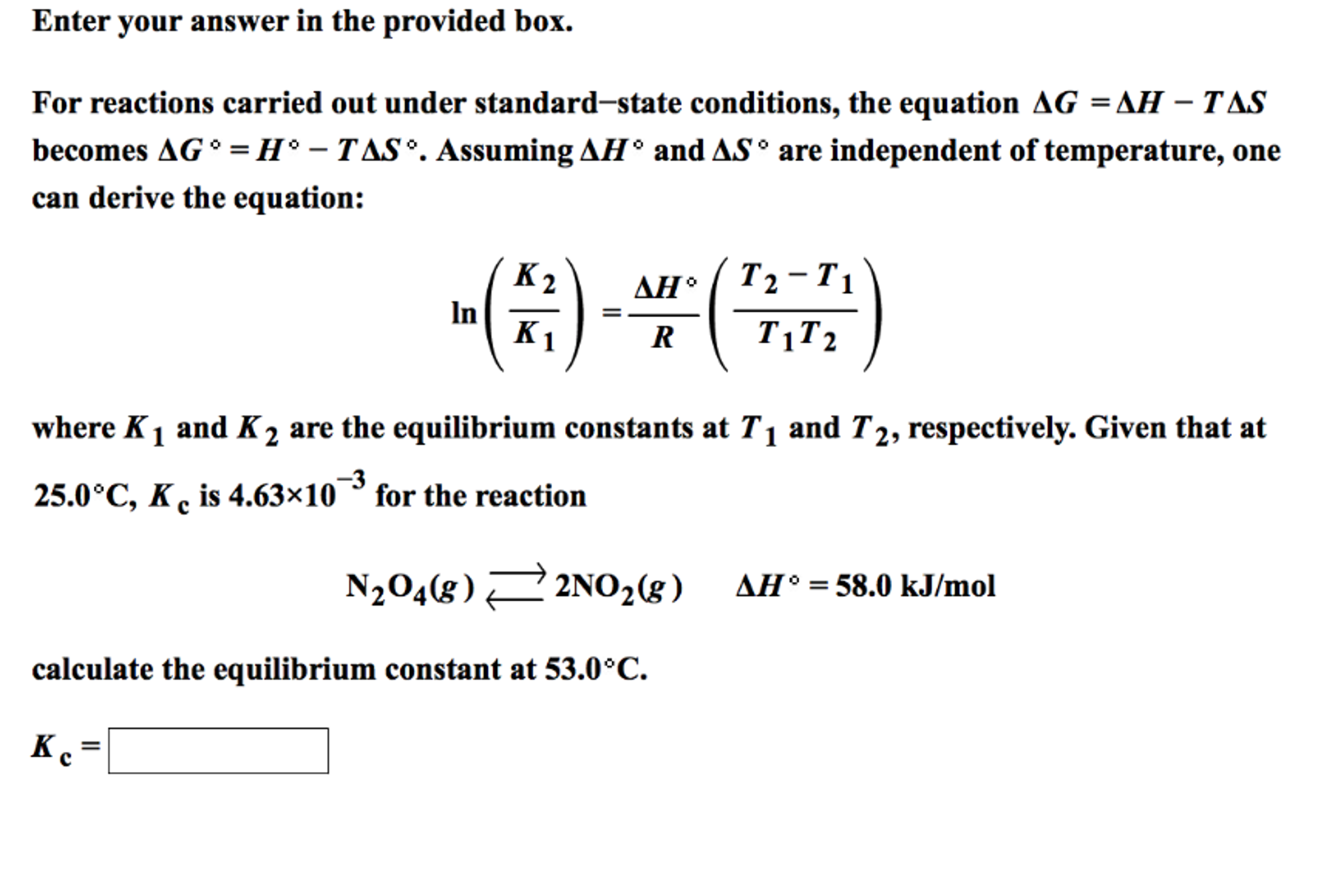
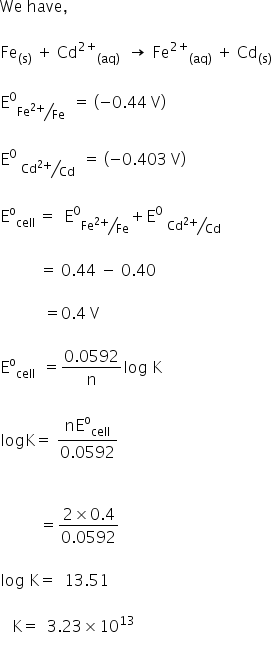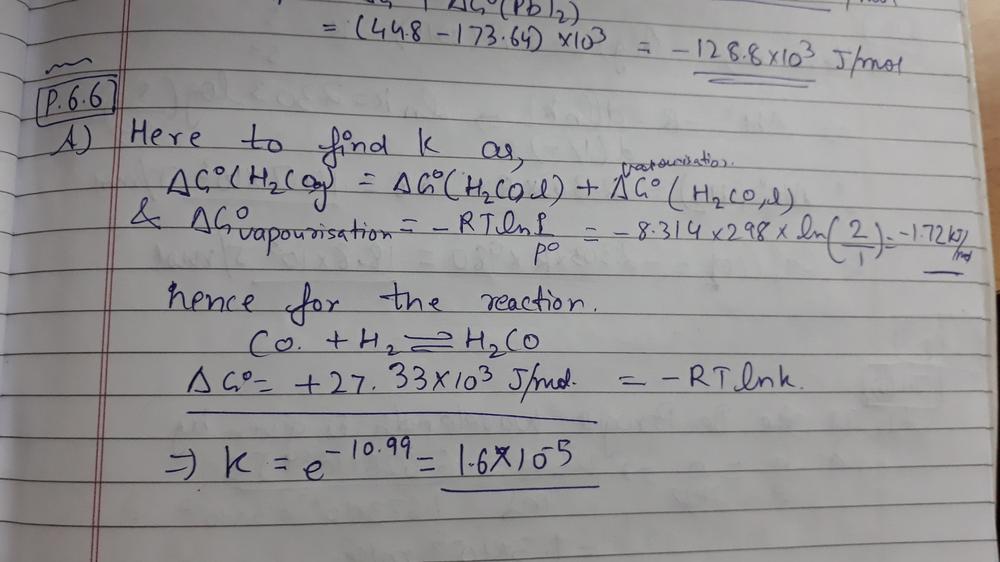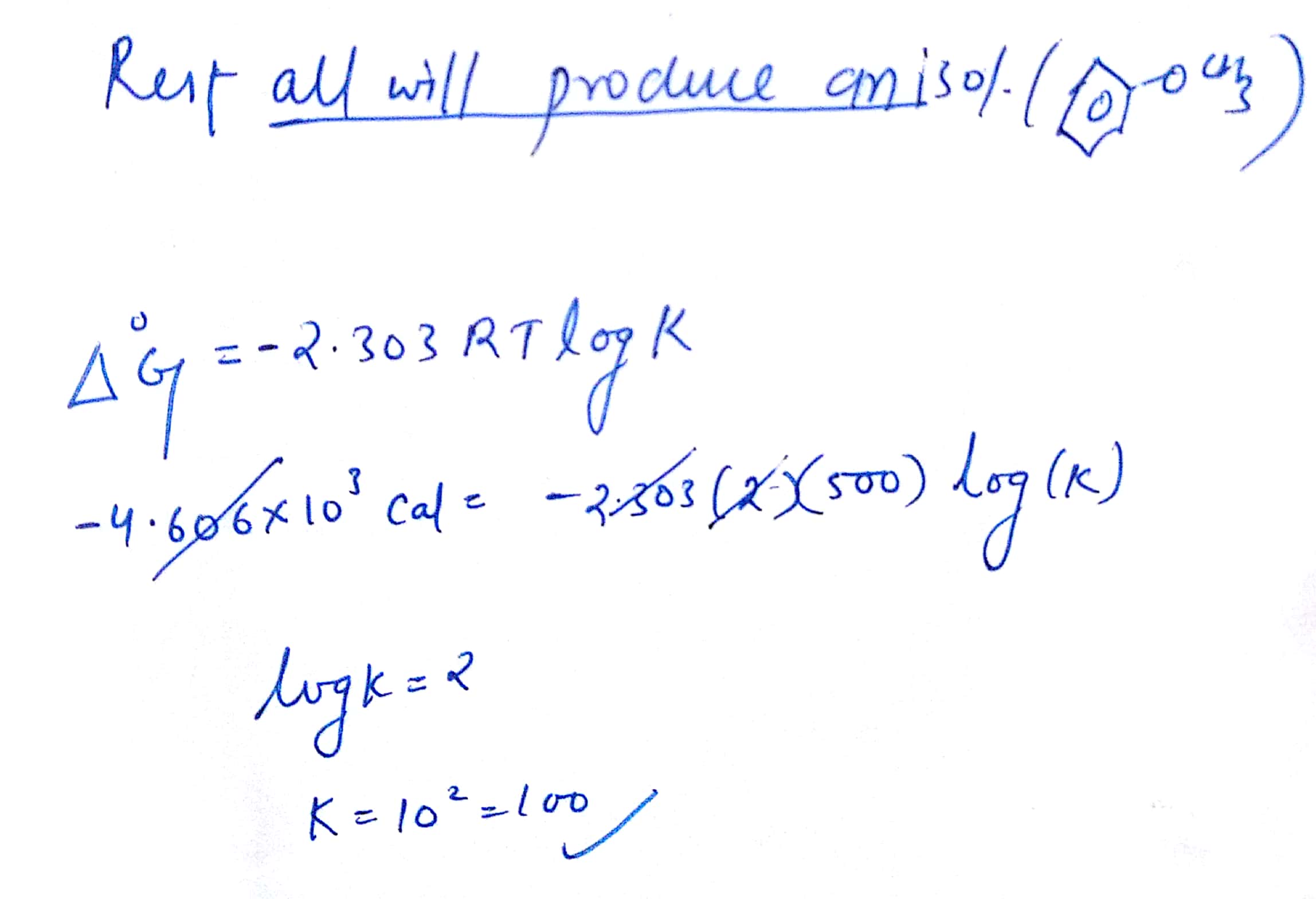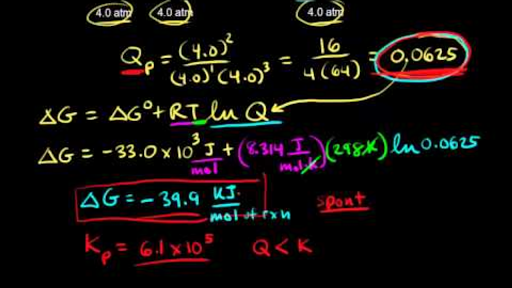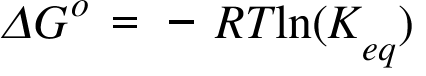How To Calculate Equilibrium Constant Given Delta G
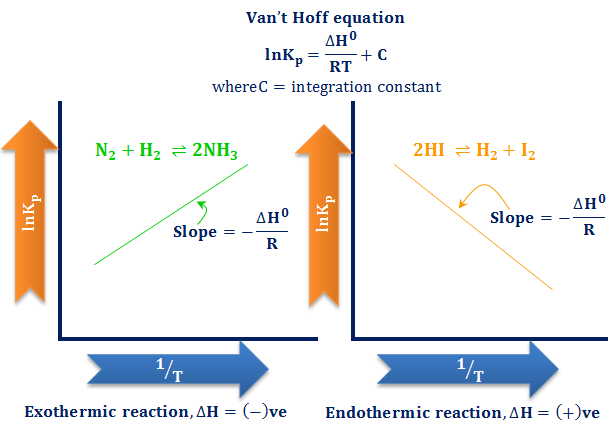
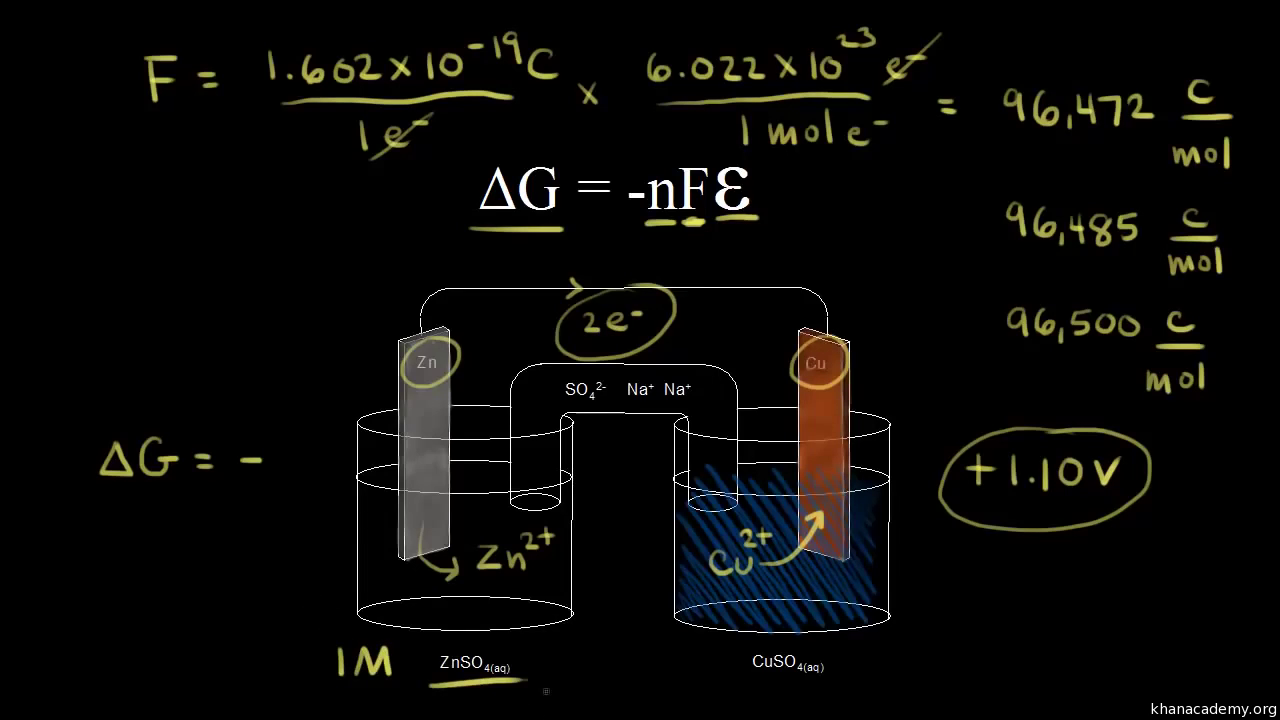
So delta g naught is constant for a given reaction.
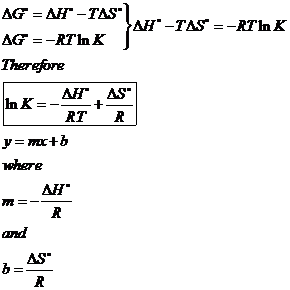
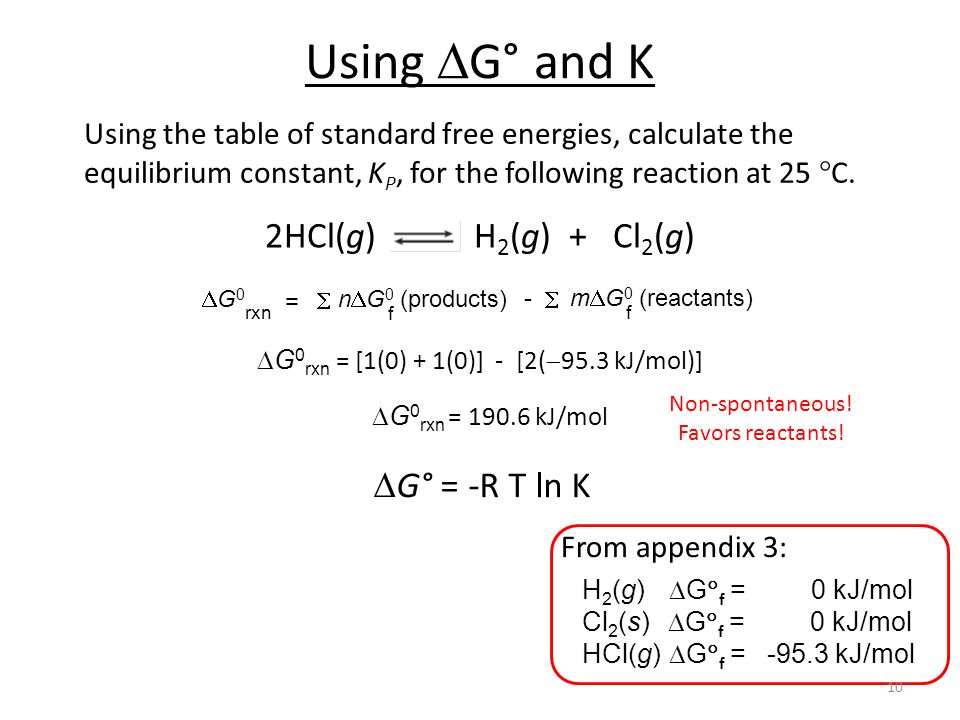
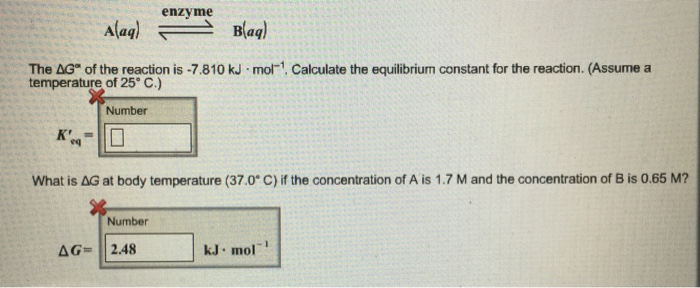
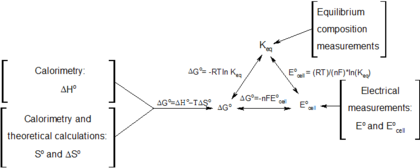
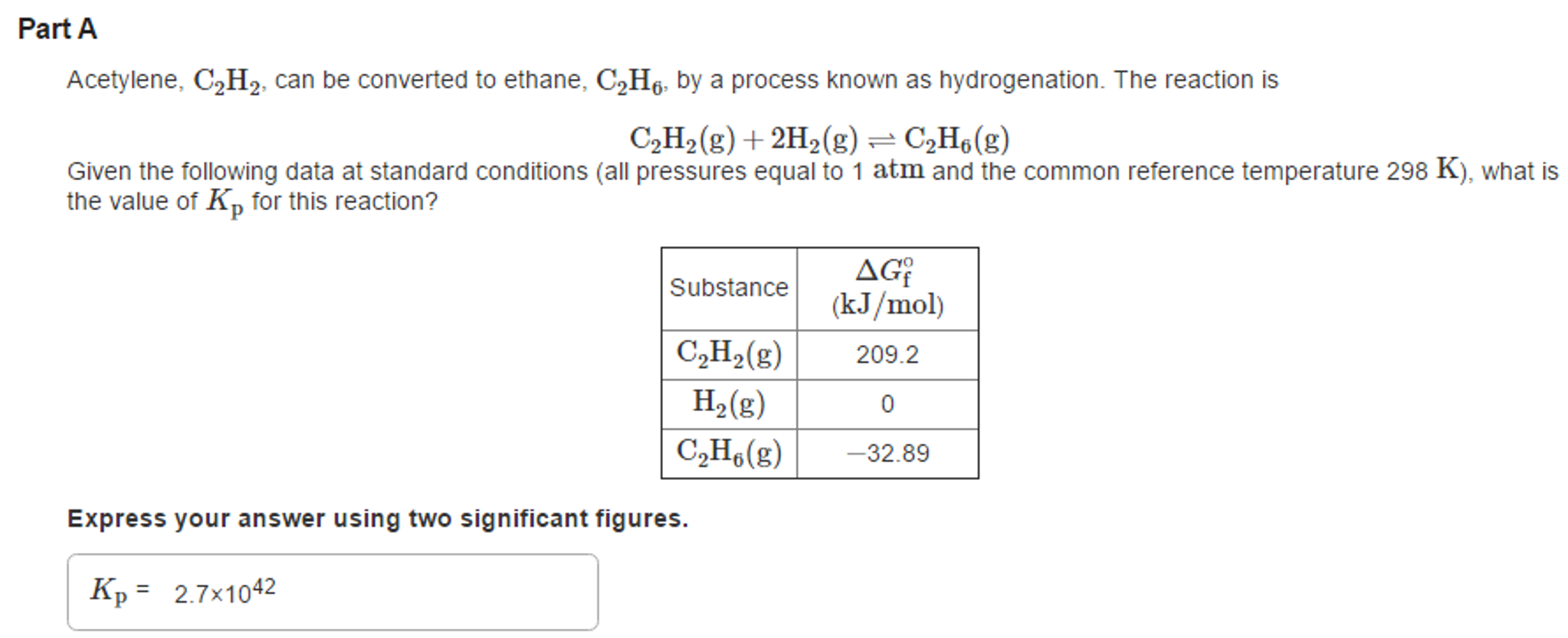
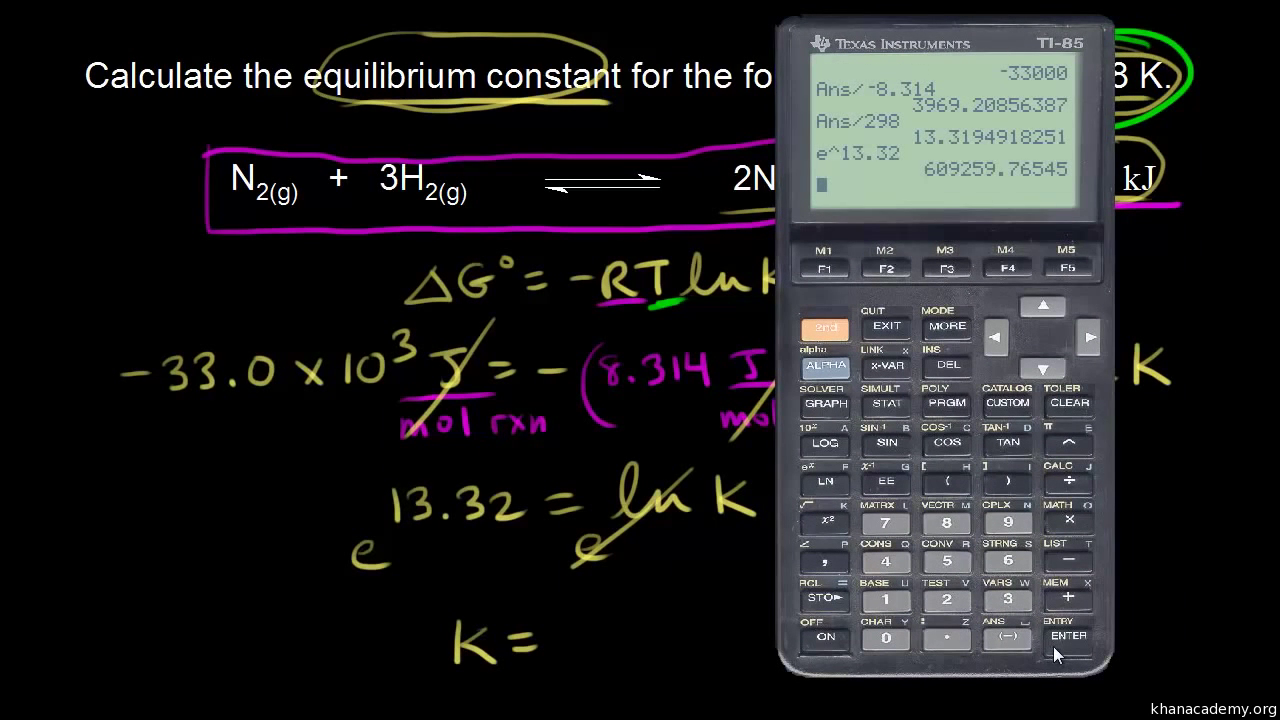
How to calculate equilibrium constant given delta g. Calculate the value of the equilibrium constant k c for the system shown if 01908 moles of co 2 00908 moles of h 2 00092 moles of co and 00092 moles of h 2 o vapor were present in a 200 l reaction vessel were present at equilibrium. Calculating equilibrium concentrations from the equilibrium constant. If we know the standard state free energy change gofor a chemical process at some temperature t we can calculate the equilibriumconstant for the process at that temperature using the relationship between goand k. But delta g naught is the delta g at standard condition.
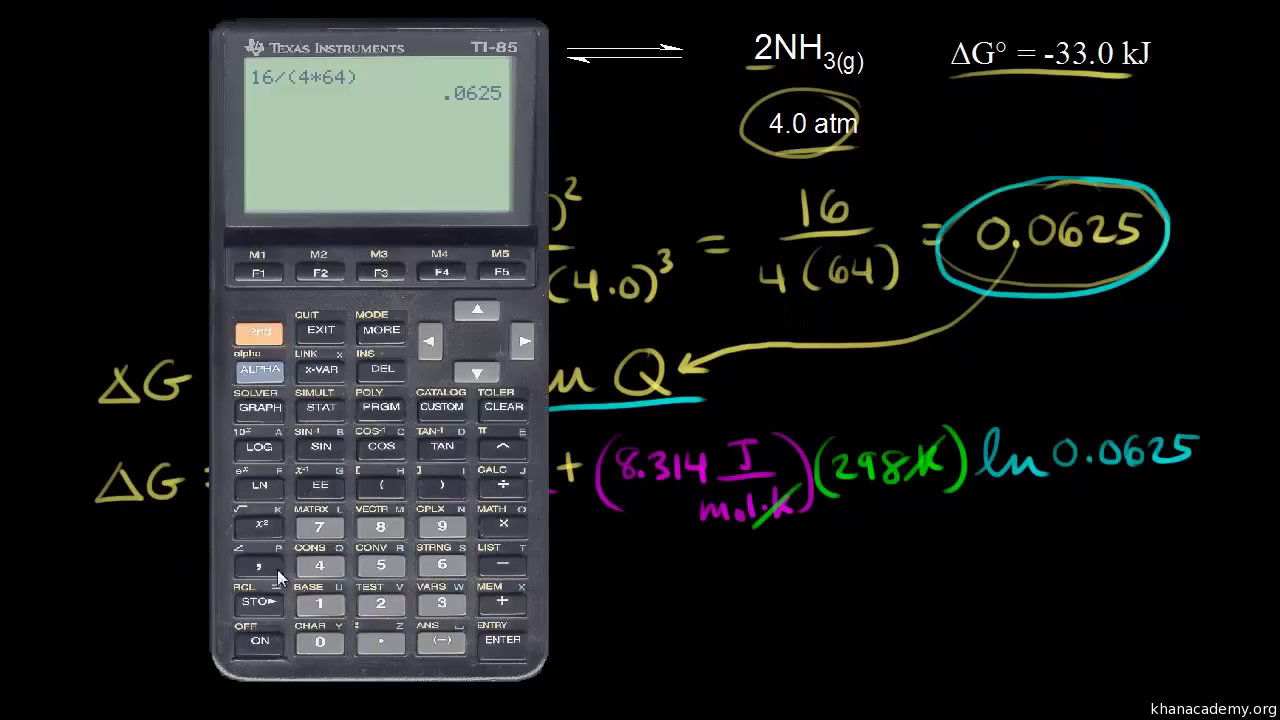
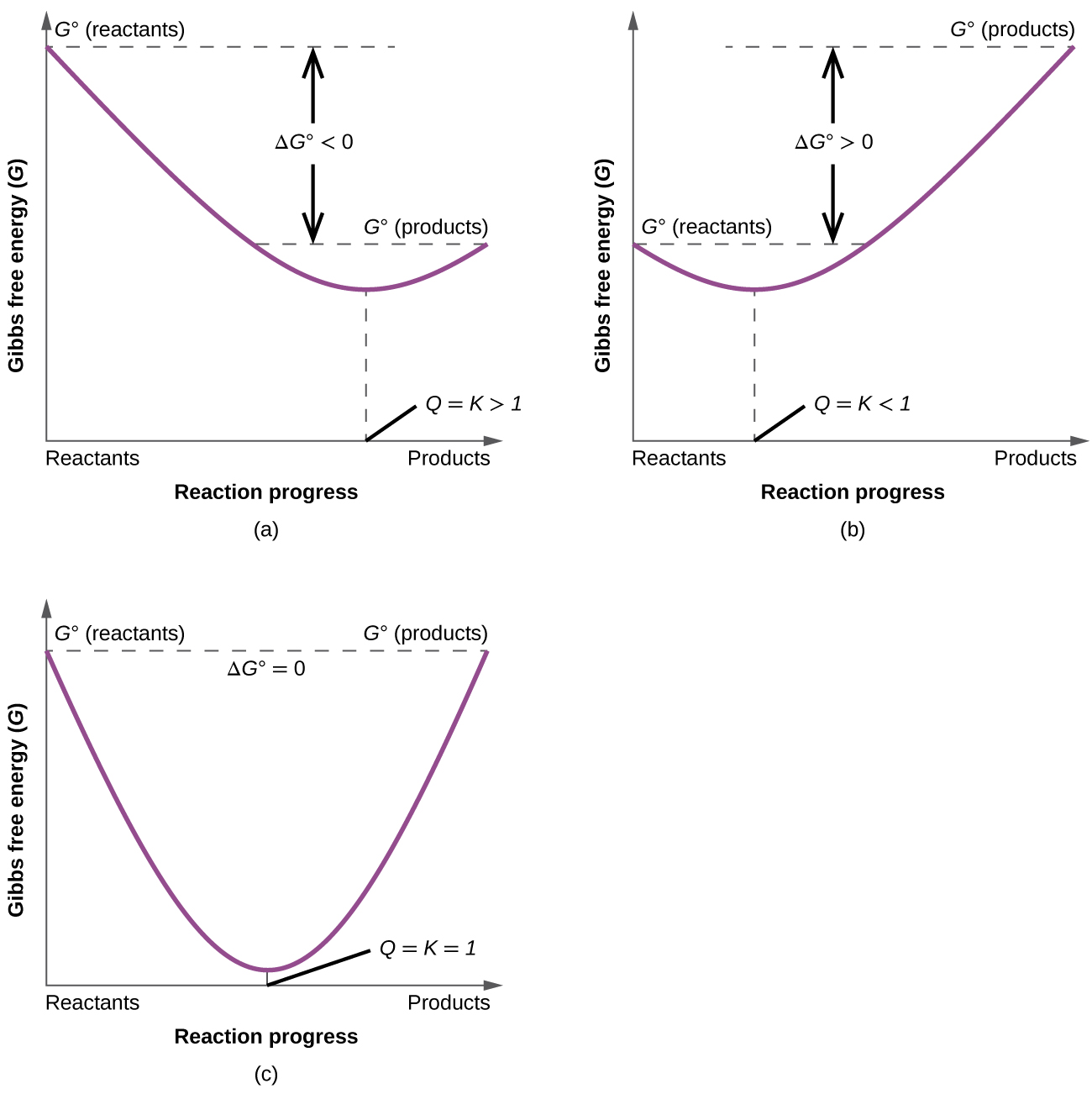
Where r is the universal gas constant 1987 calmol t is the absolute temperature and q is the reaction quotient. So k the equilibrium constant is equal to 27 times ten to the negative six. Conversely if dg0 0 then k p 1 and reactants are favored over products when the reaction is at equilibrium. Alright so k is less than one.
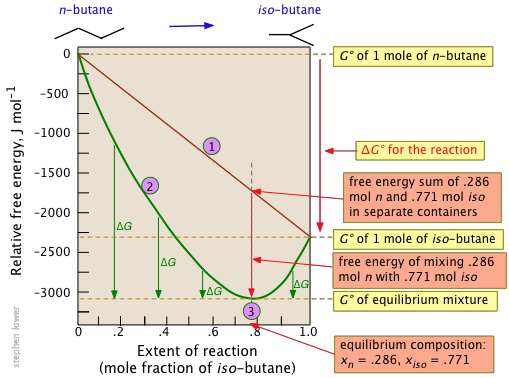
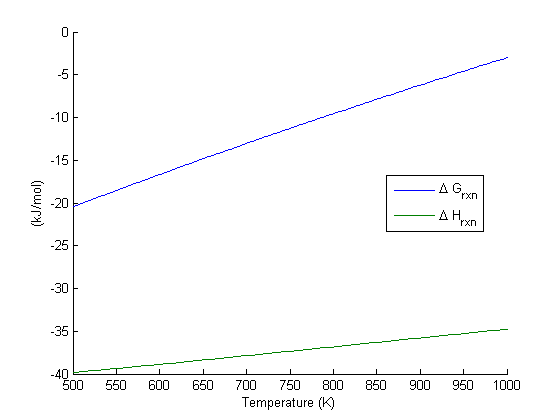
If the products and reactants are in their standard states and dg0 0 then k p 1 and products are favored over reactants when the reaction is at equilibrium. To describe how to calculate equilibrium concentrations from an equilibrium constant we first consider a system that contains only a single product and a single reactant the conversion of n butane to isobutane equation refeq1 for which k 26 at 250c. 25 c 1 atm. So when delta g zero is greater than zero so when its positive your equilibrium constant k is less than one.
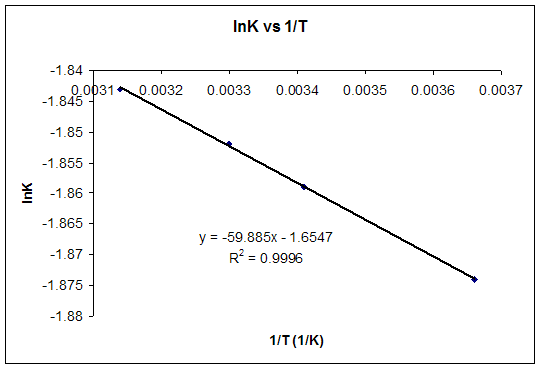
Equilibrium constant from delta g. So when delta g zero is positive when the standard change in free energy is positive lets write this one down. 19711 d g 0 r t ln.