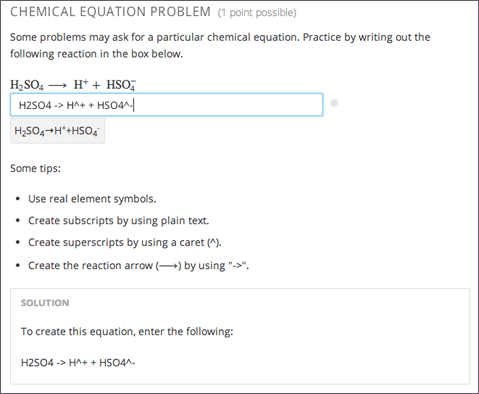
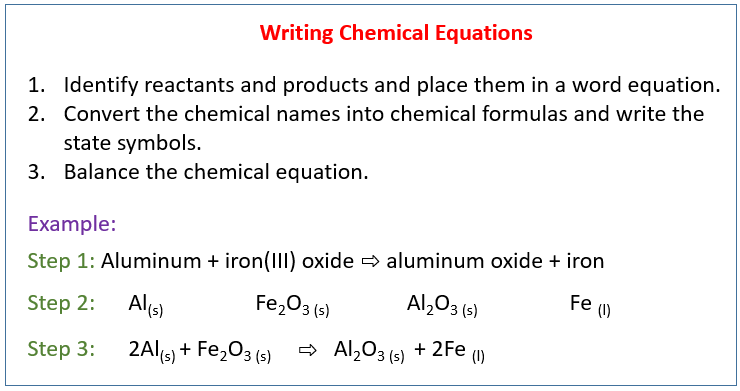
How To Balance Chemical Equations Examples
For this example you will use.
:max_bytes(150000):strip_icc()/84288434-58b5b38e3df78cdcd8add154.jpg)
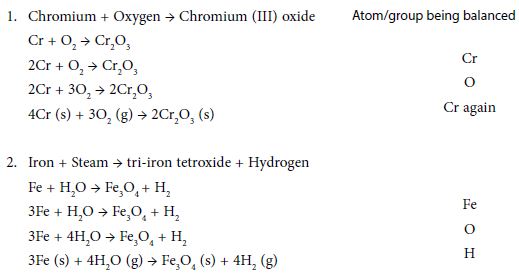
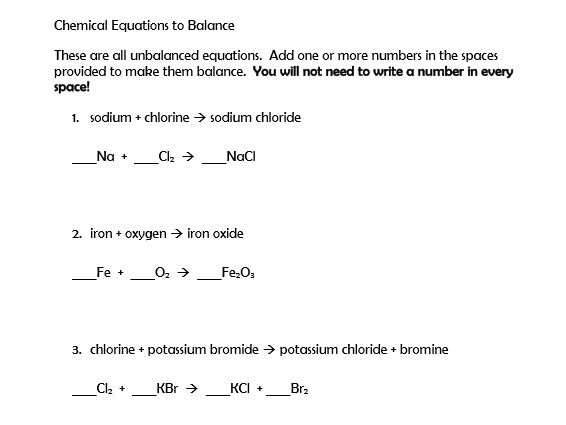
How to balance chemical equations examples. Use the cho technique. Add up the numbers of each type of atom. Look at the subscripts next to each atom to find the number of atoms in the equation. Write the number of atoms 3.
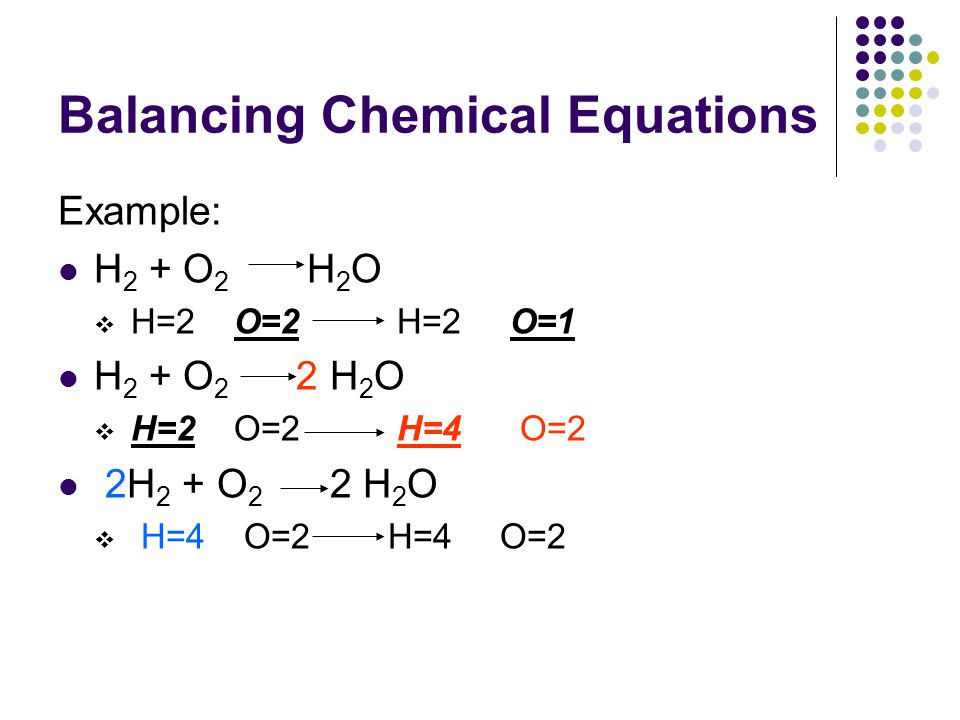
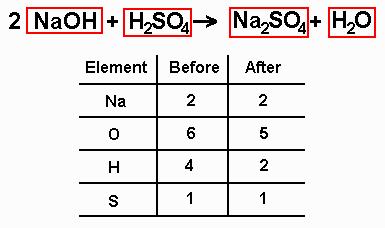
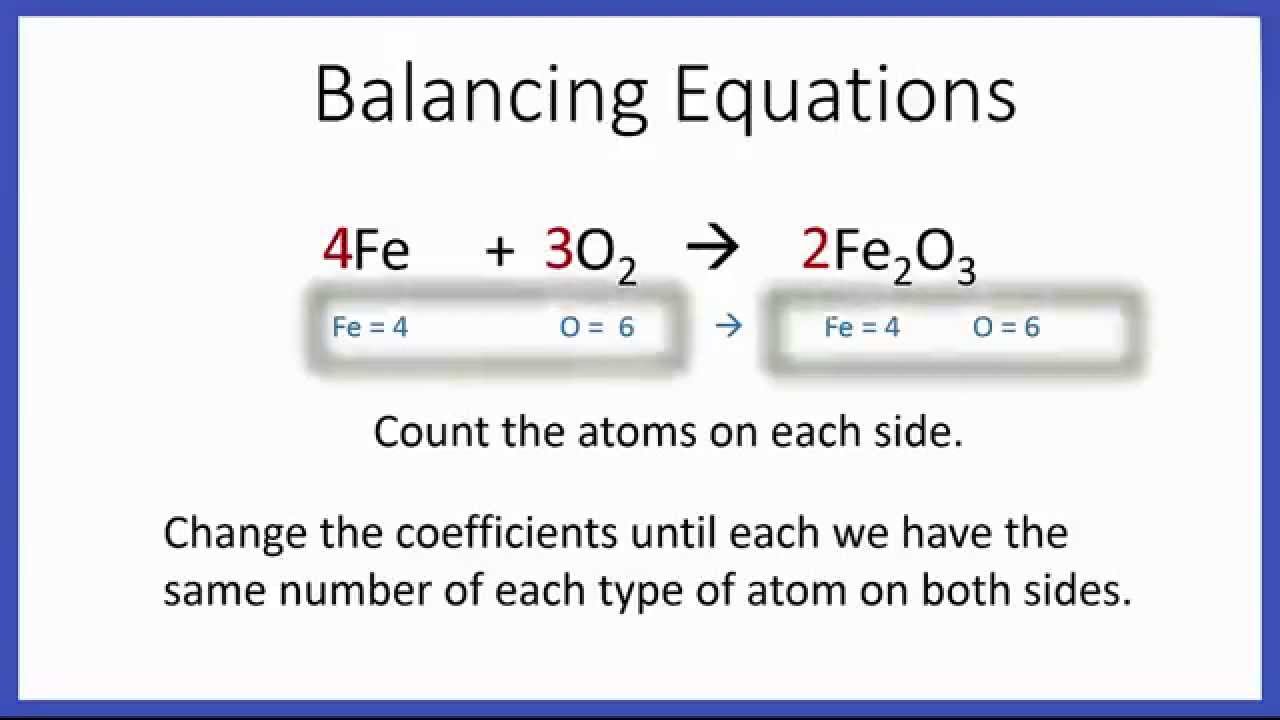
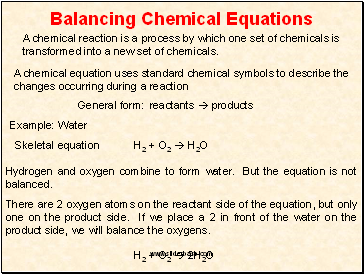
In the example equation there are two atoms of hydrogen on each side but there are two atoms of oxygen on the left side and only one on the right side. When writing it out its a good idea to connect it back to the original equation noting how each element. We have five hydrogen atoms on the left and three hydrogen atoms on the right. Balance the chemical equation.
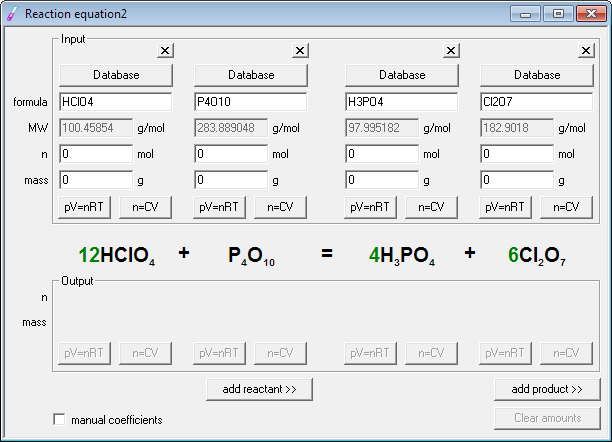
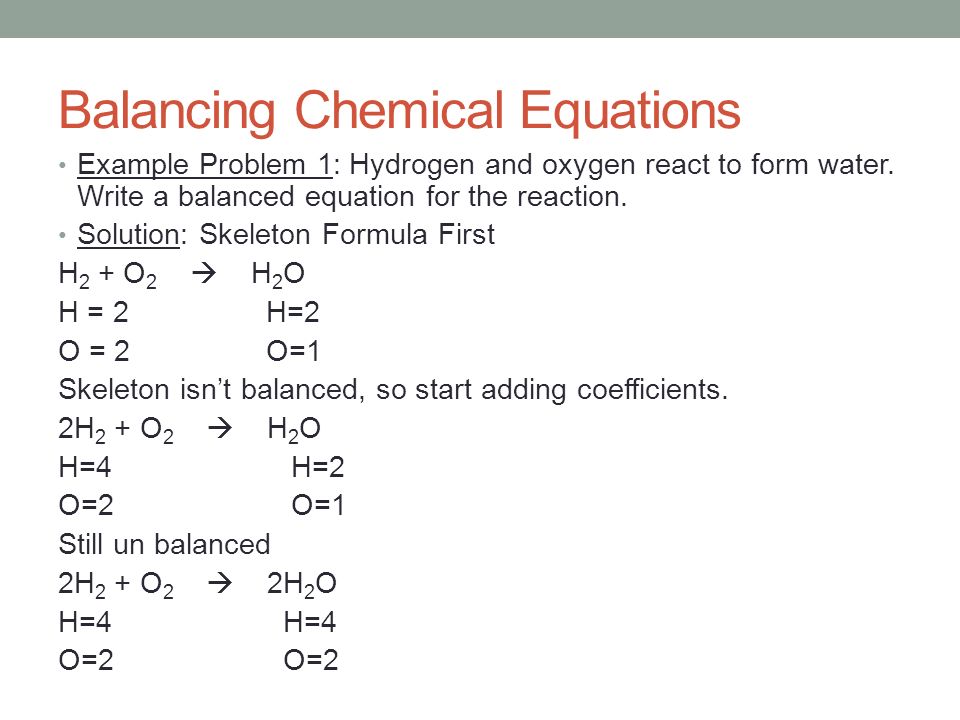
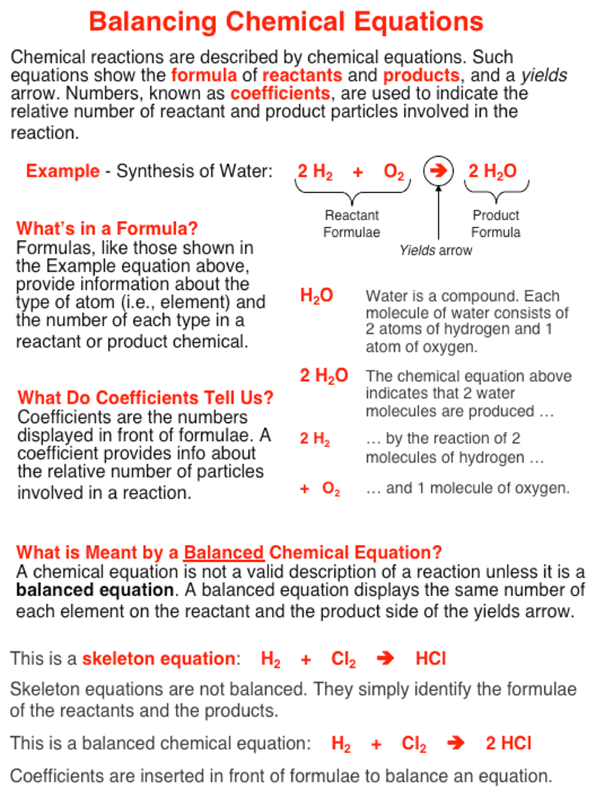
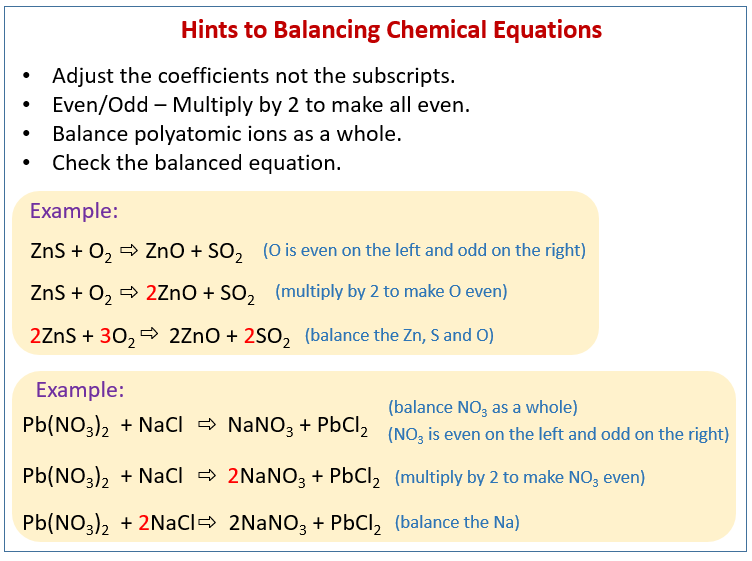
Step 1 write down your given equation. Remember thisa balanced equation must have equal numbers of each type of atom on both sides of the arrow. When you balance a chemical equation its always a good idea to check the final equation to make sure it works out. How to balance chemical equationsexplanation and example 1.
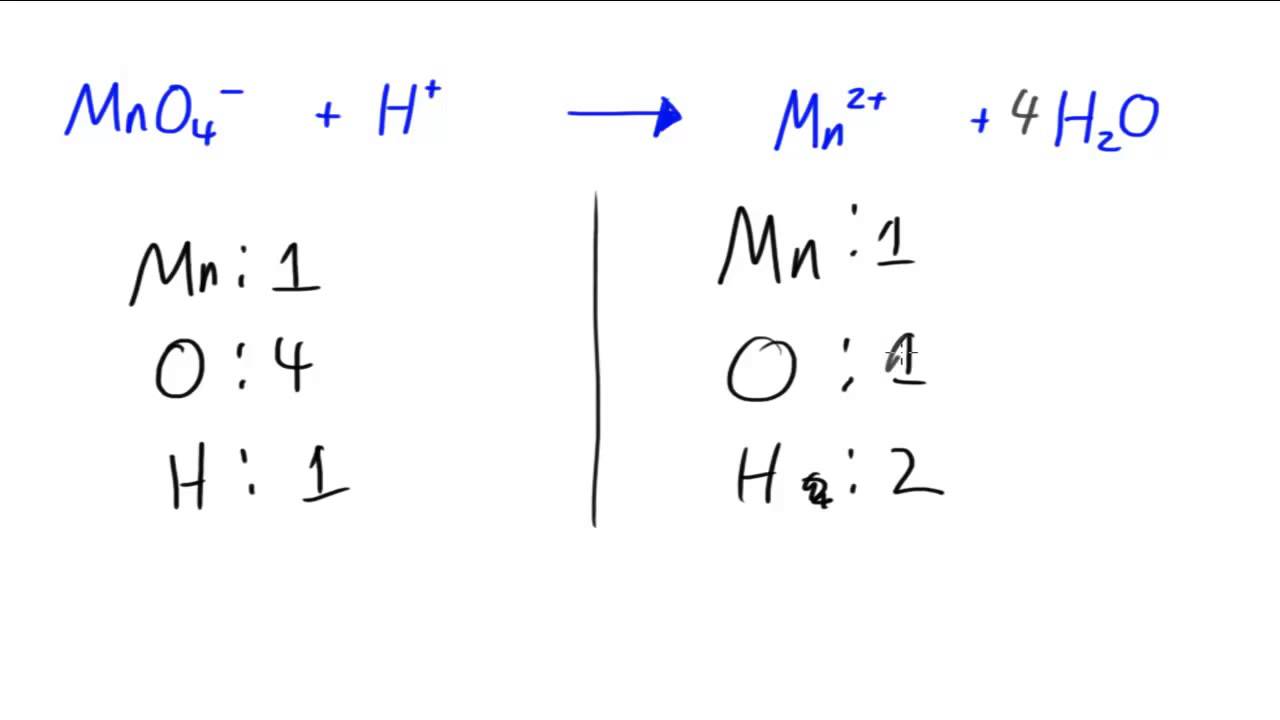
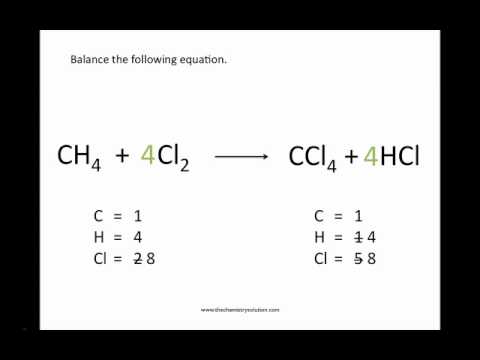
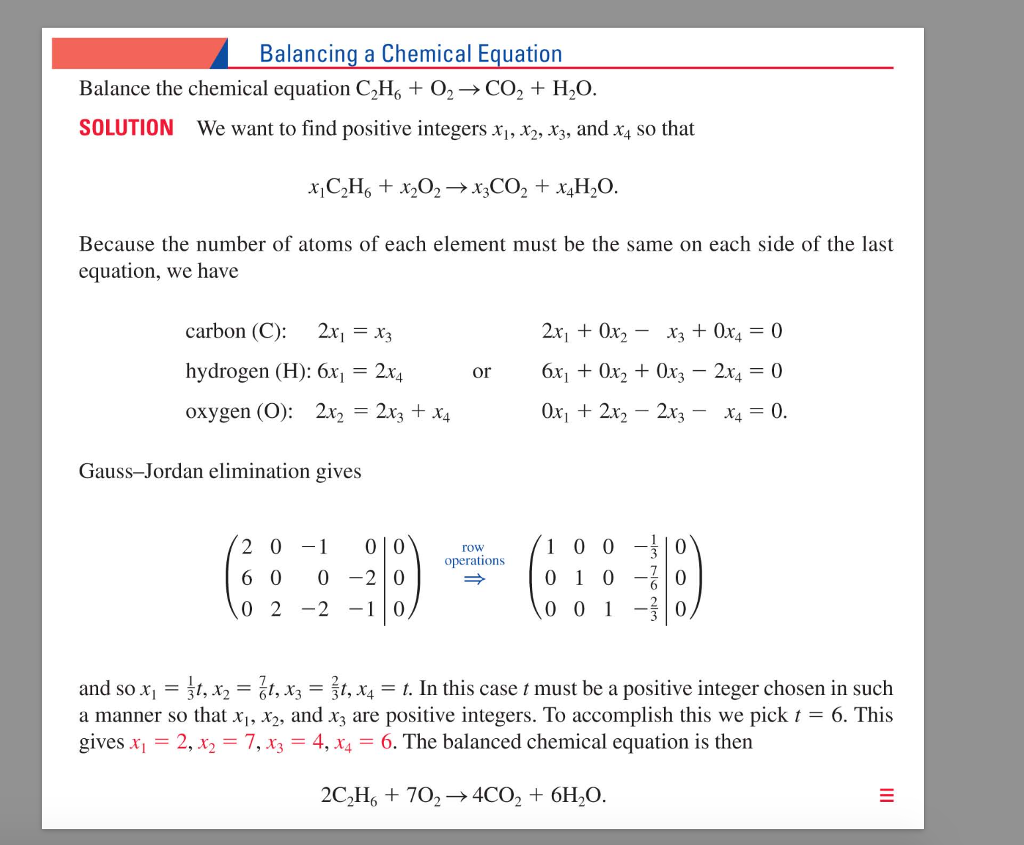
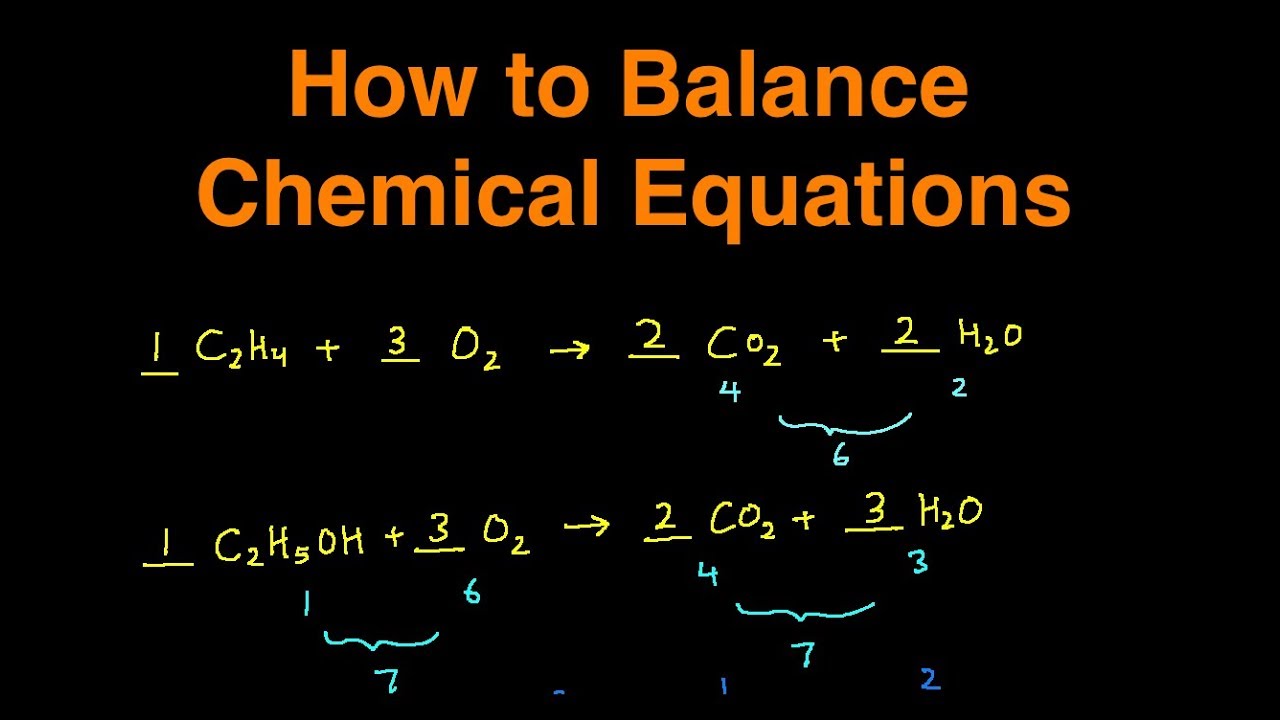
Put a 5 in front of the co 2 on the right hand side. There are five carbons on the left but only one on the right and on each side the carbon is in a single chemical species. Do this for each side of the equation. Check equations to make sure they are balanced.
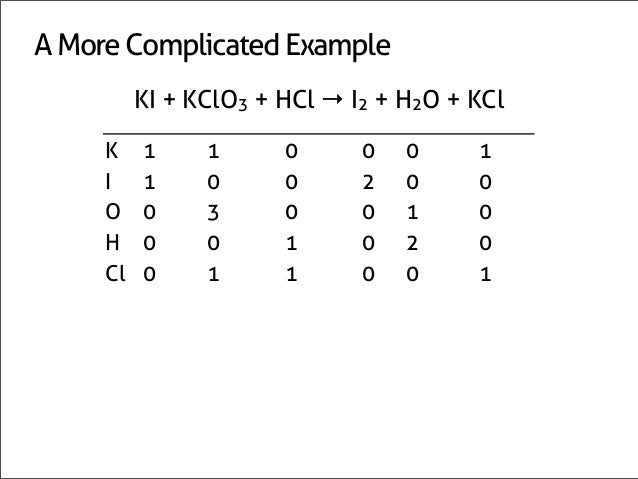
The total number of atoms in a balanced equation will be the. Identify the products and reactants 2. The following figure gives some hints on how to balance chemical equations. Since we dont have carbon we could try to balance the hydrogen first.
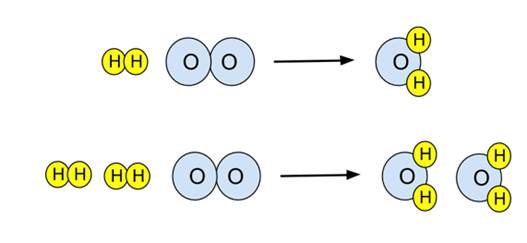
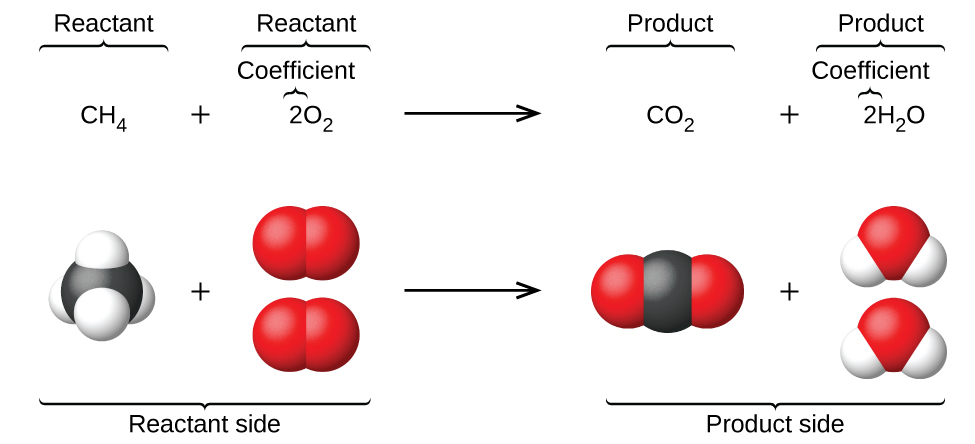
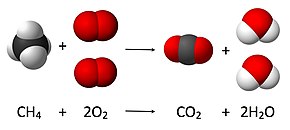
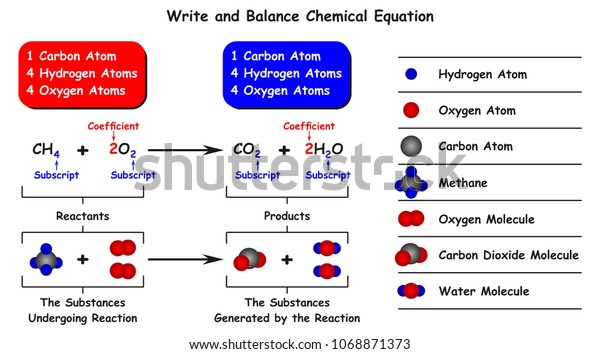
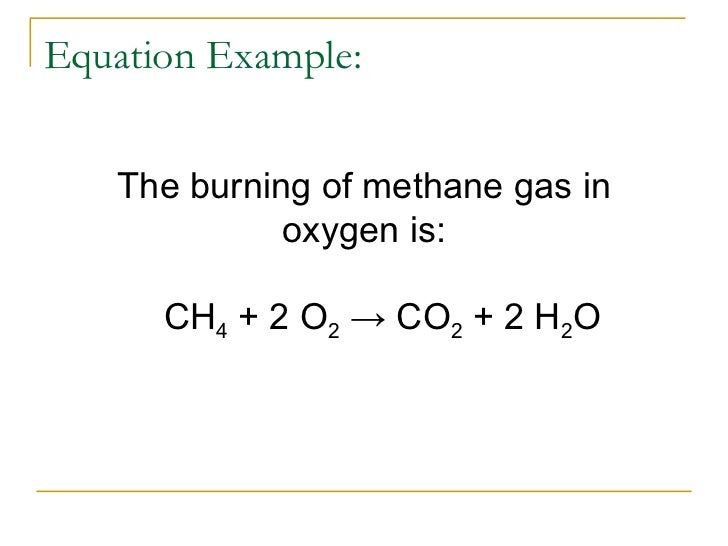
Introduction to balancing chemical equations in this video well start out with examples that show the concepts behind balancing chemical equations. How to balance chemical equations. There are twelve hydrogens on the left but only two on the right hand side and hydrogen is in a single species on each side. C3h8 o2 h2o co2 this reaction occurs when propane c3h8 is burned in the presence of oxygen to produce water and carbon dioxidestep 2 write down the number of atoms per element.
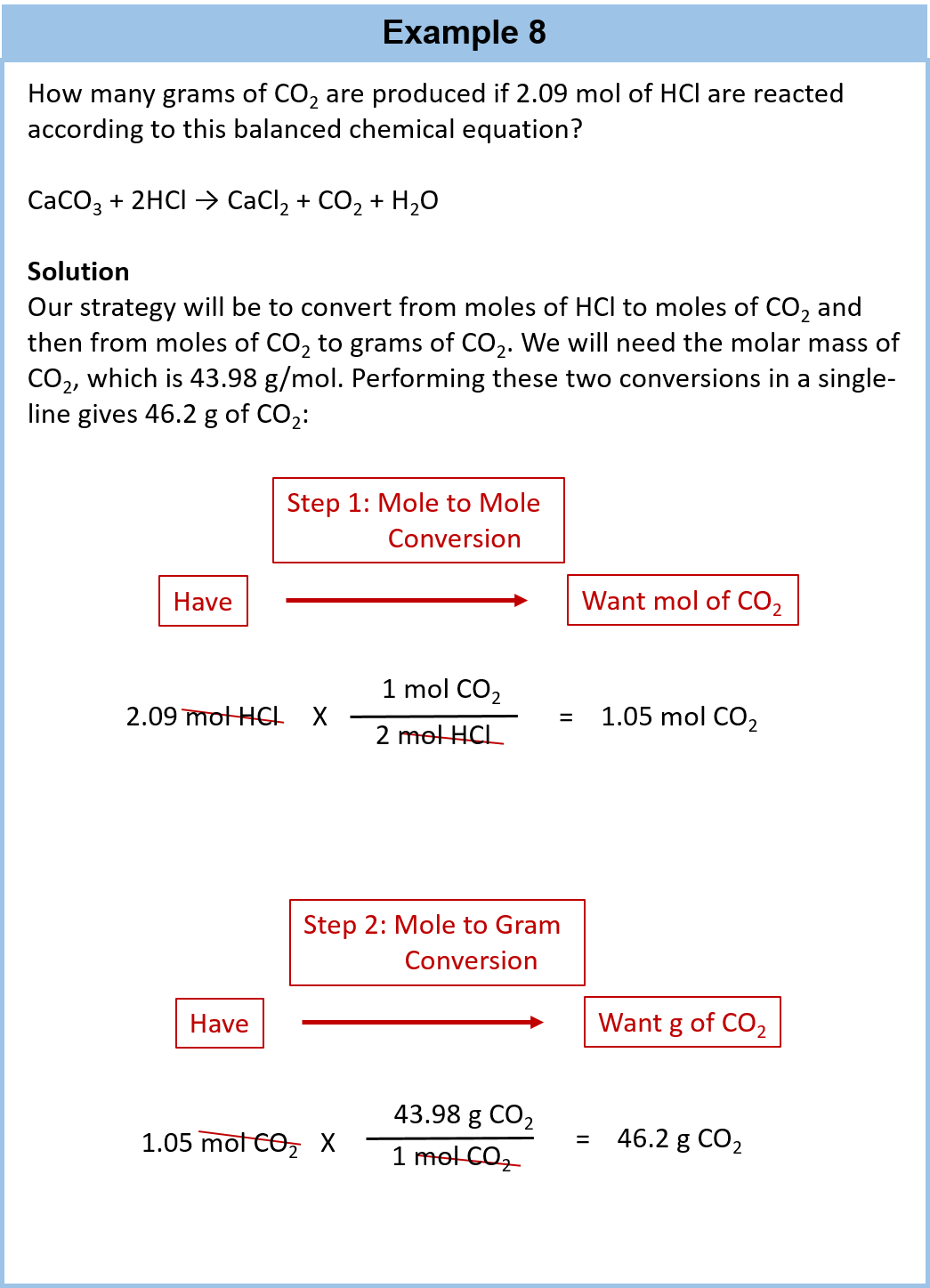
Scroll down the page for more examples and solutions. An equation is balanced by changing coefficients in a somewhat trial and error fashion.






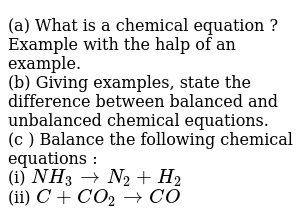




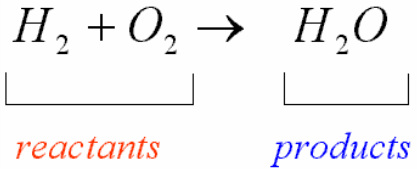



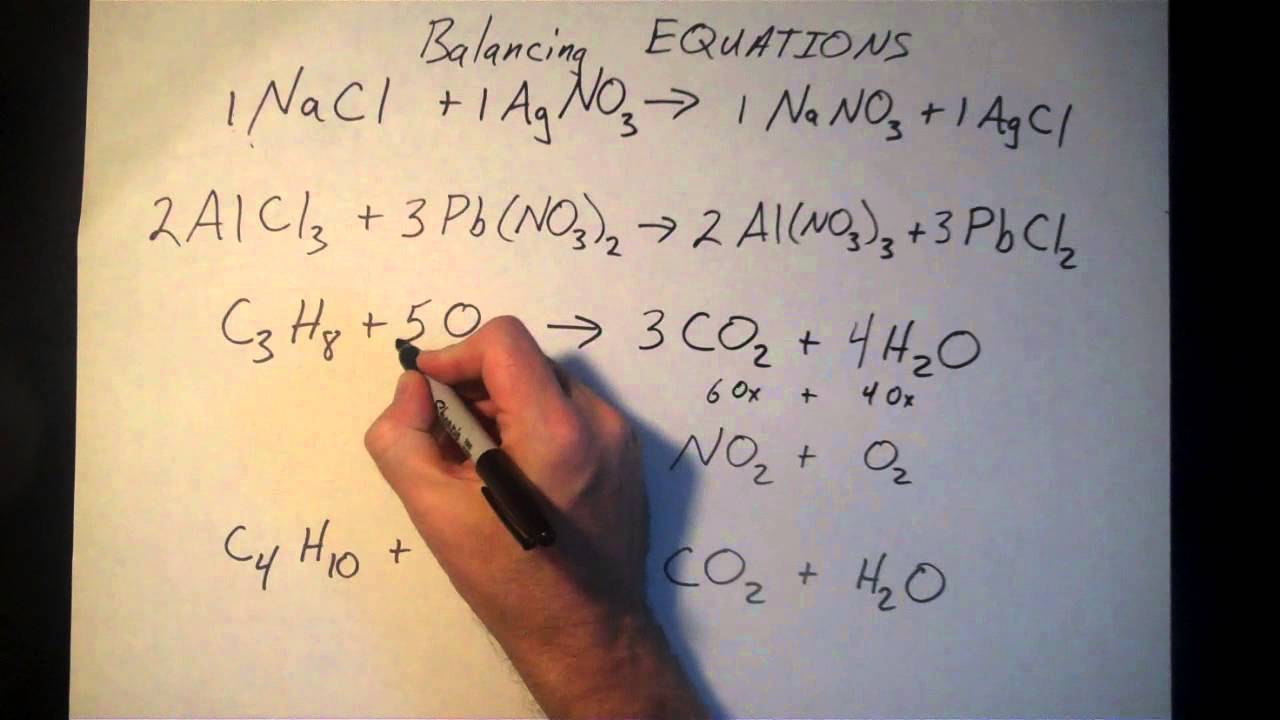















.PNG)































/chemicalequations-58ea60c53df78c516211b81d.jpg)