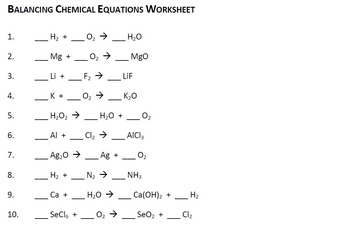
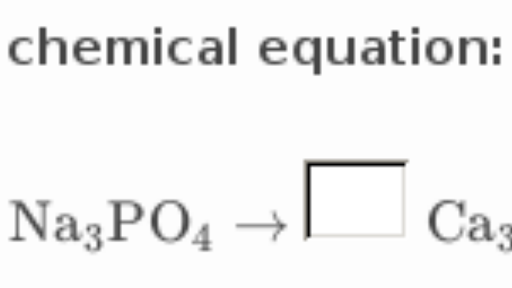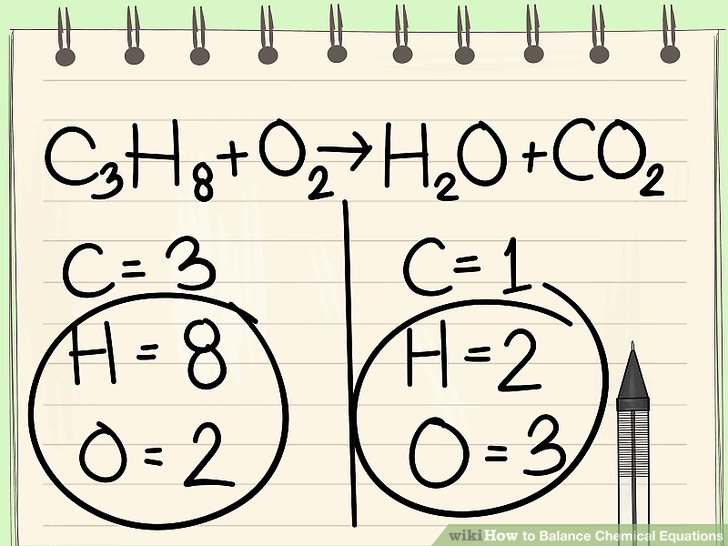How To Balance Chemical Equations Easily
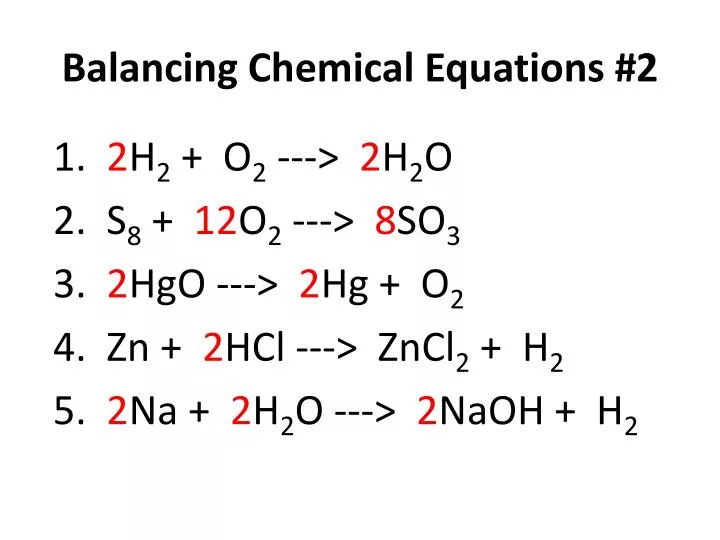
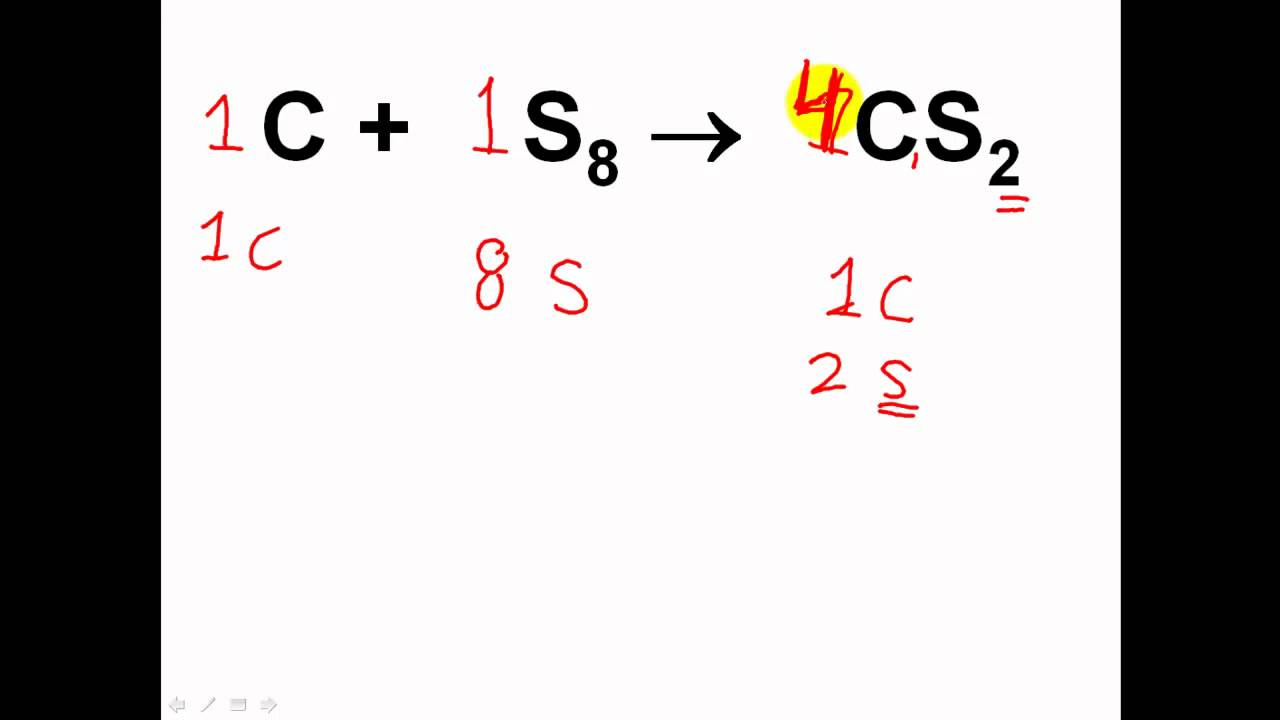
For example your equation should look something like h2 o2 h2o count the number of atoms in each element on each side of the equation and list them under that side.
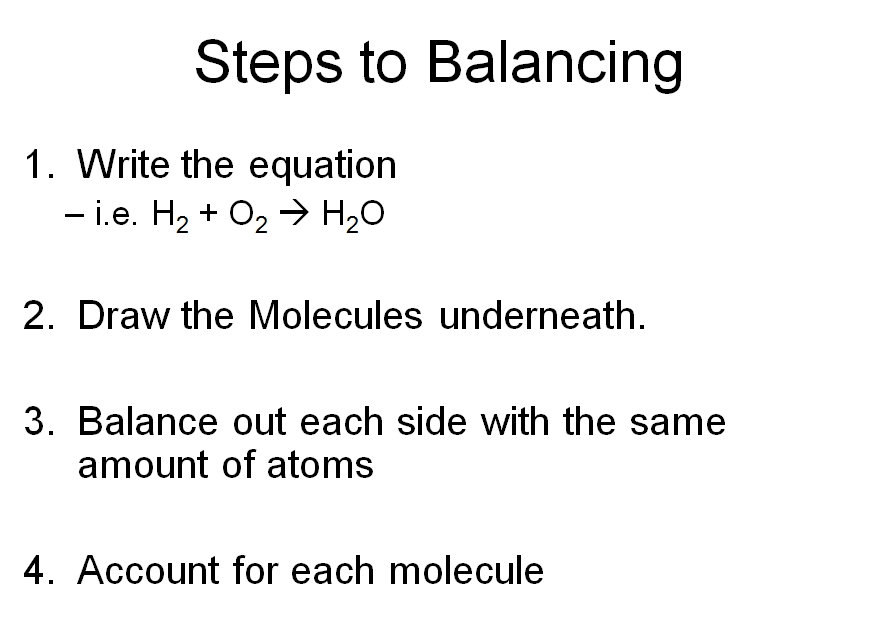
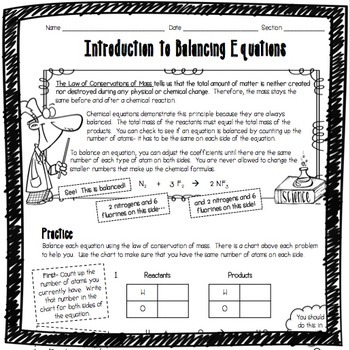
How to balance chemical equations easily. If possible start. The first step is to write down the unbalanced chemical equation. Write down number of. The number of atoms of each type of atom must be the same on each side of.
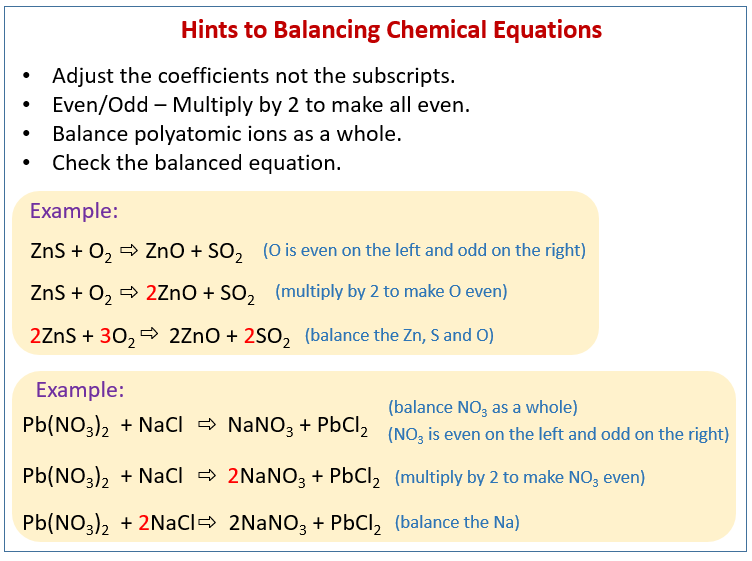
There are three key things to remember when balancing reactions. There are no rules that explain how you get a balanced reaction from expression. How to balance chemical equationsexplanation and example 1. Write the number of atoms 3.
How to balance chemical equations easy steps for balancing chemical equations. But it is hard to find atomic oxygen. You know the reactants and the product for this reaction and you cant change them. The net charge must be the same on each side of the equation once.
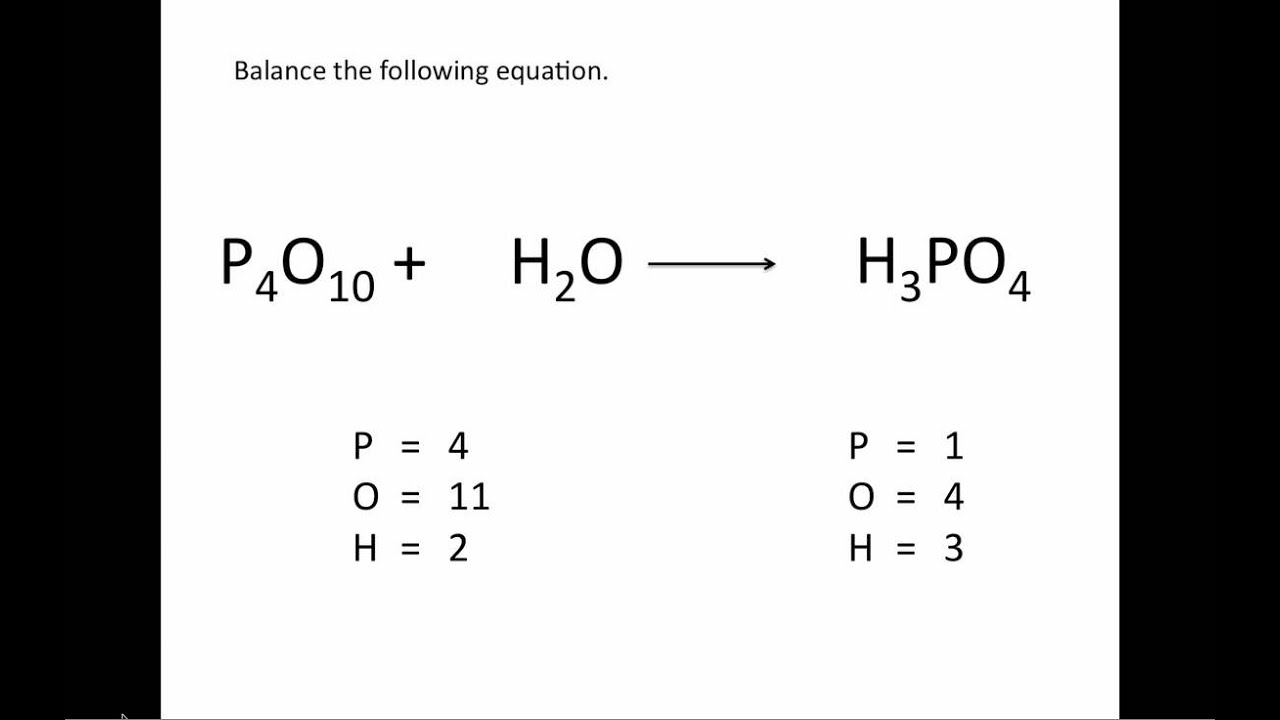
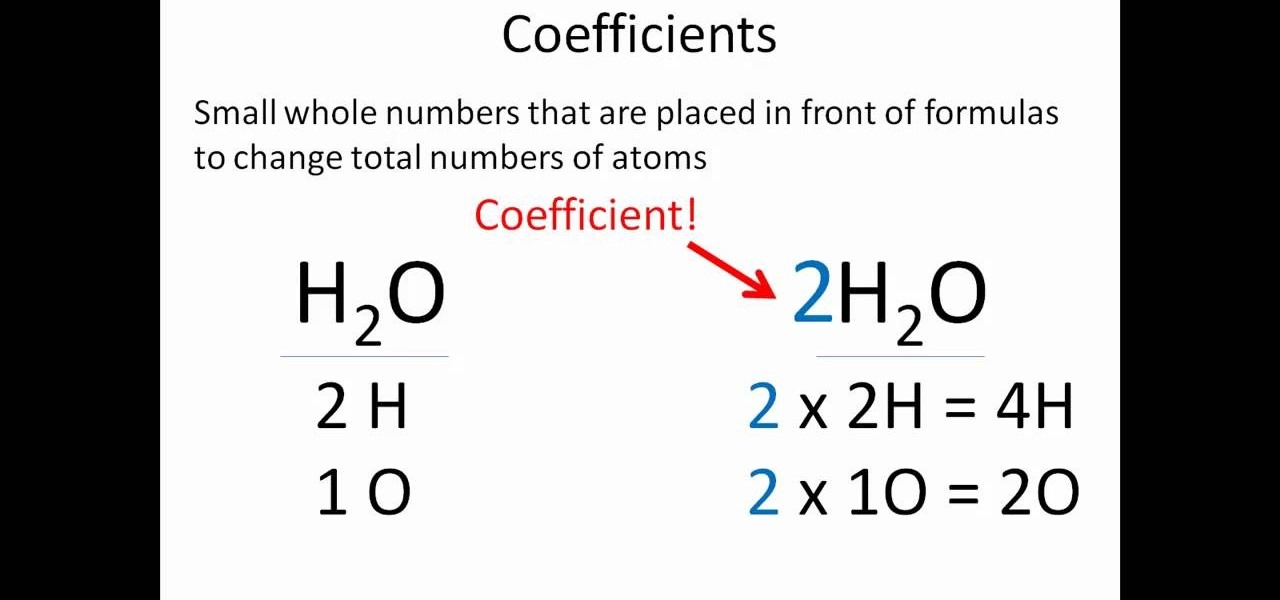
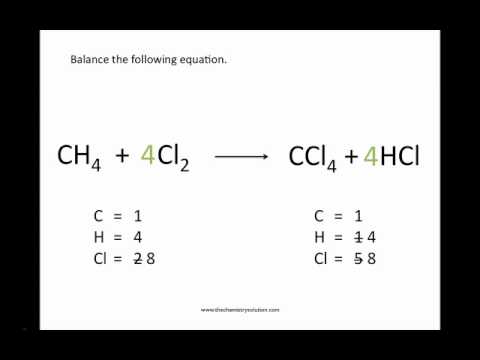
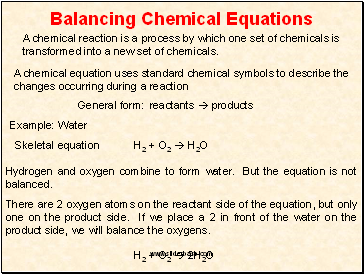
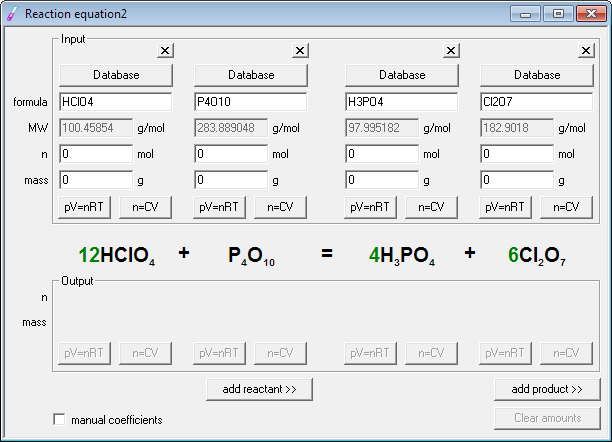
Write the unbalanced chemical equation. Hence we represent it as molecule o2. In this video we learn how to easily balance a chemical equation. To find out how much of each element you need you have to balance the equation make sure that the number of atoms on the left side of the equation equals the number of atoms on the right.
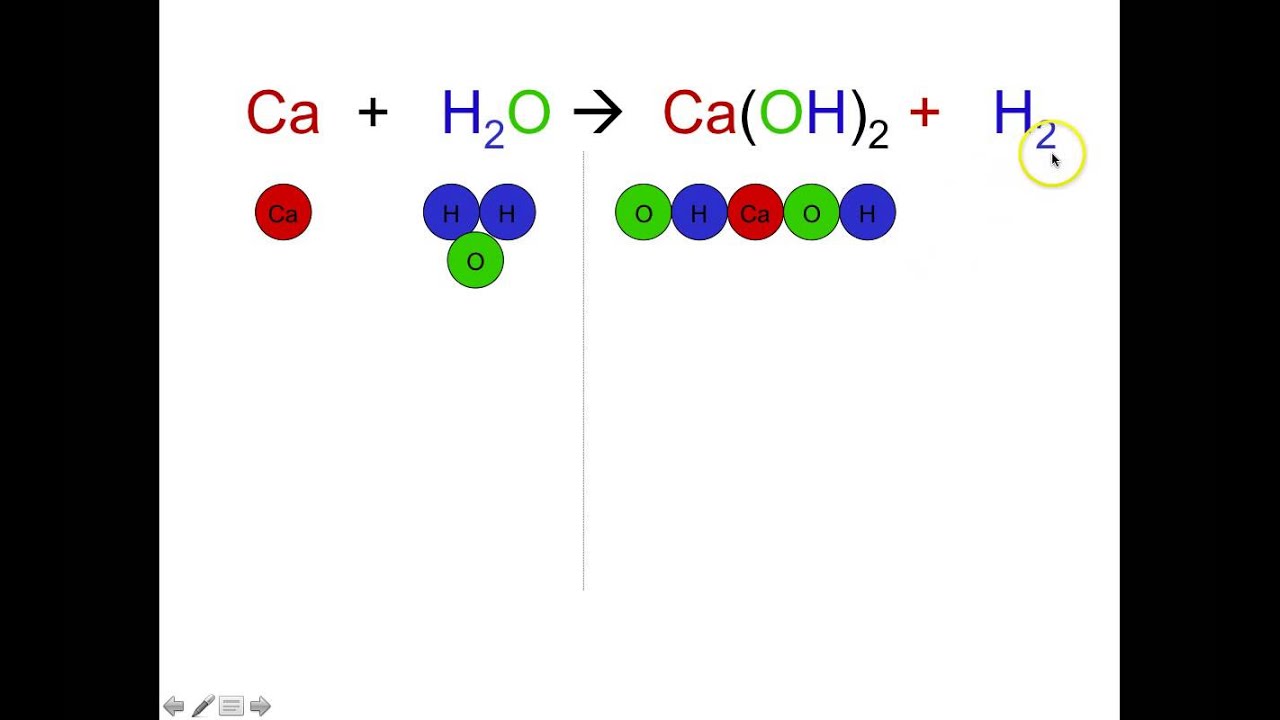
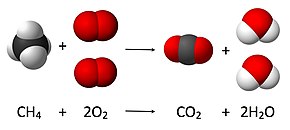
After you look at this you can learn how to make the rest of the equation equal to. For example in the above reaction one molecule of hydrogen h2 and an atomic form of oxygen o are sufficient to produce a molecule of water h2o. Identify the products and reactants 2. Steps of balancing a chemical equation identify each elementfound in the equation.
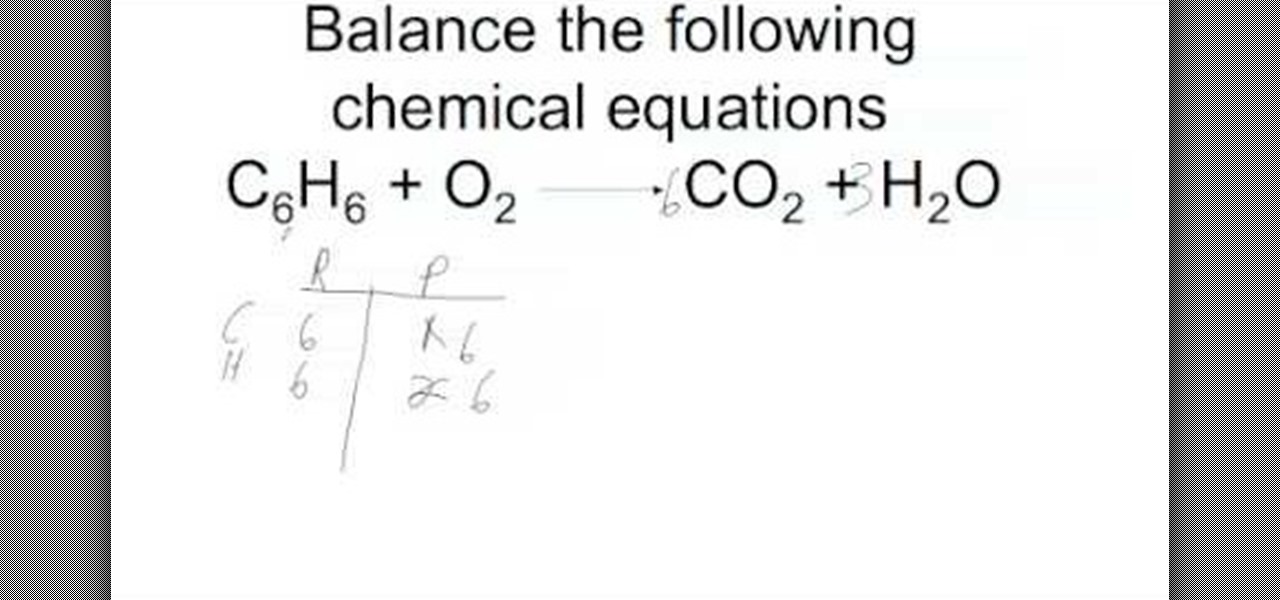
First start on the molecule or compound that is the most atomically complex. When you write a chemical equation a necessary step is to balance the number of atoms on both sides of the equation. A chemical equation is a written description of what happens in a chemical. It helps to make a table below the equation to.
Learn how to balance chemical equations here. To balance a chemical equation first write out your given formula with the reactants on the left of the arrow and the products on the right. What is the net charge on each side of the equation.
Https Encrypted Tbn0 Gstatic Com Images Q Tbn 3aand9gcreyr31tyucpqq0ehmedl8uqs5gvavfvkui 1unzn 027x7vr8p Usqp Cau


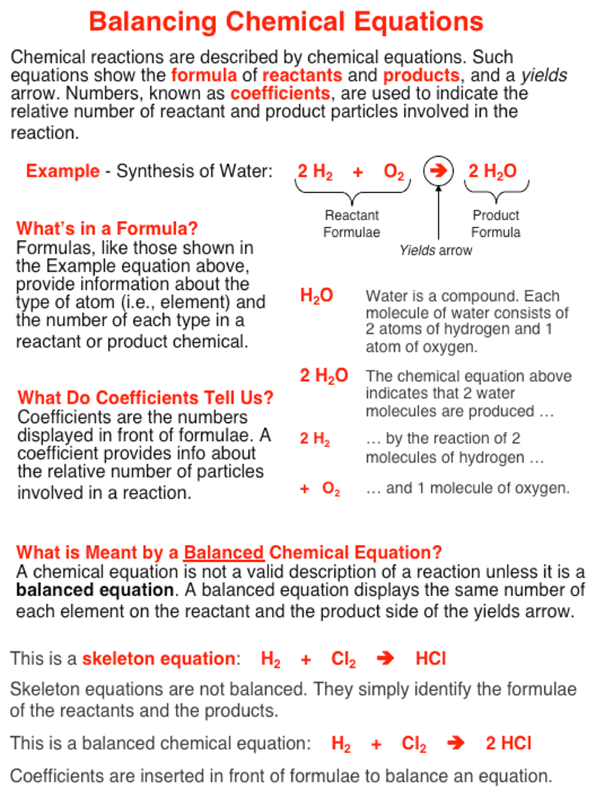












































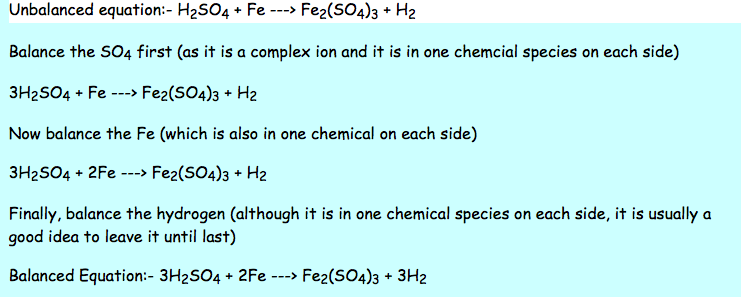













:max_bytes(150000):strip_icc()/84288434-56a130f53df78cf772684635.jpg)