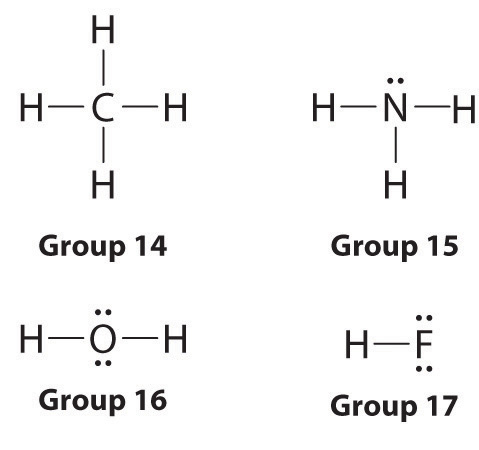
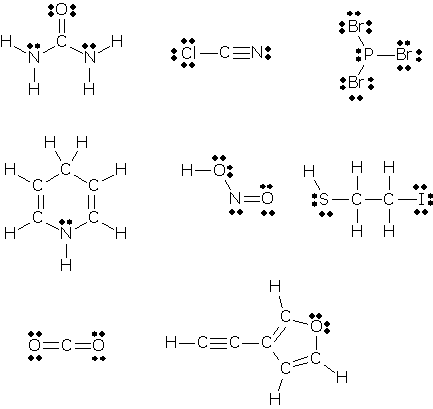
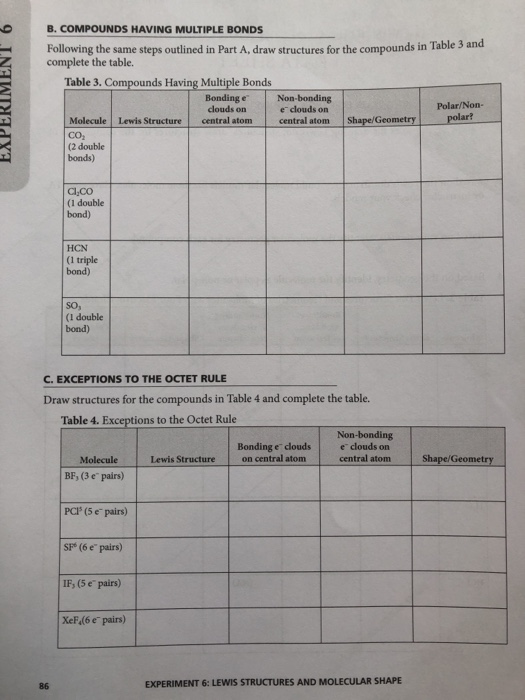
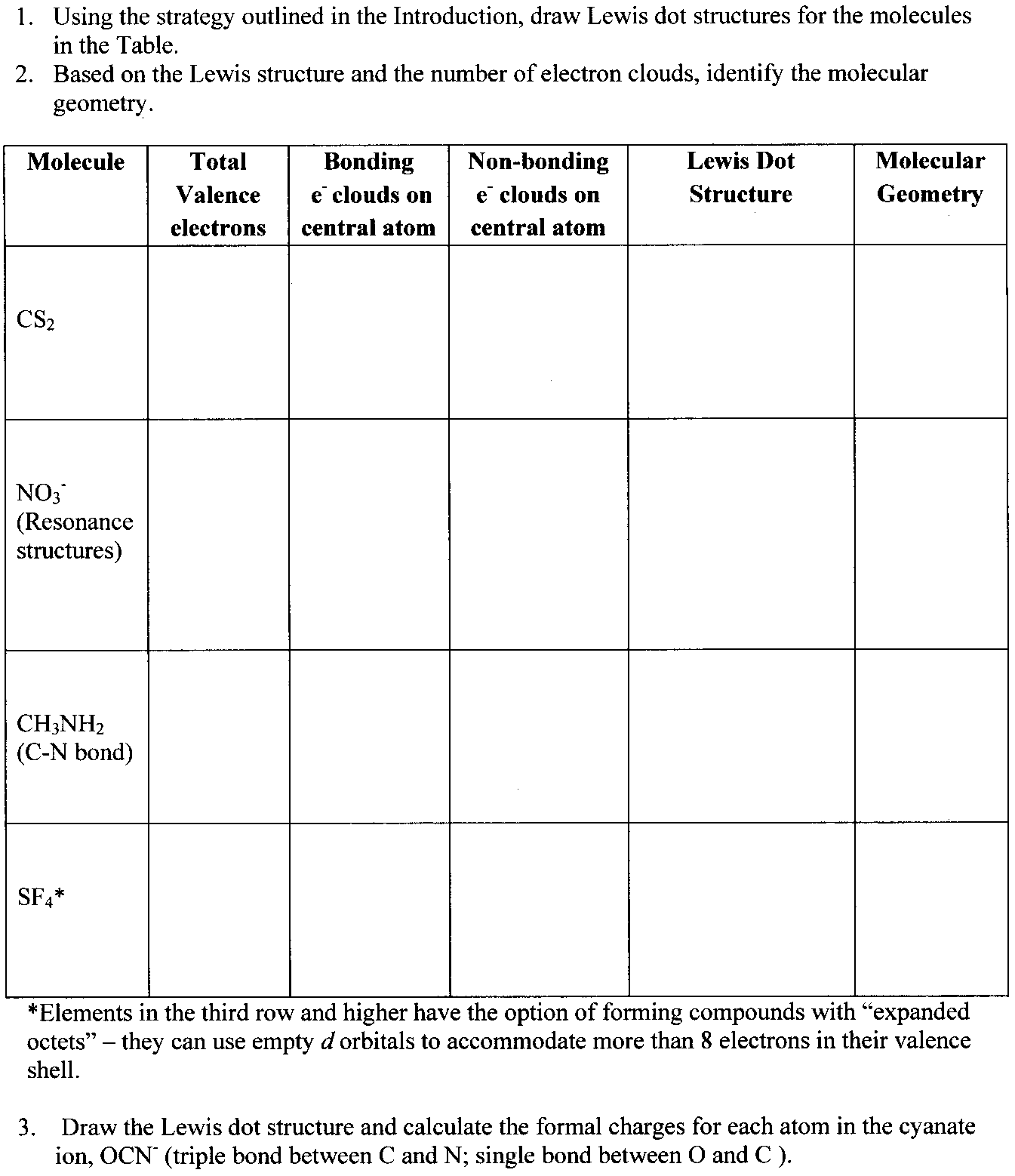
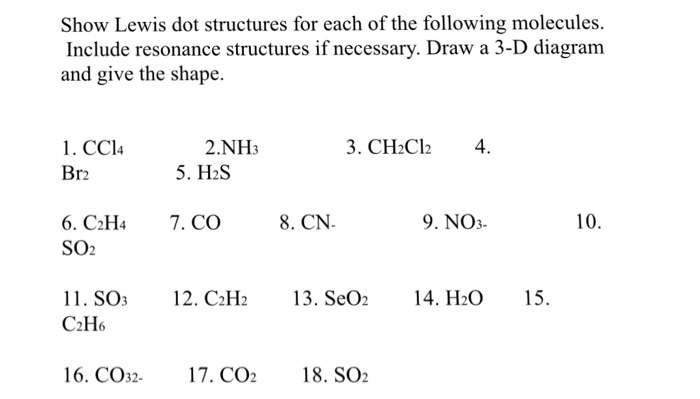
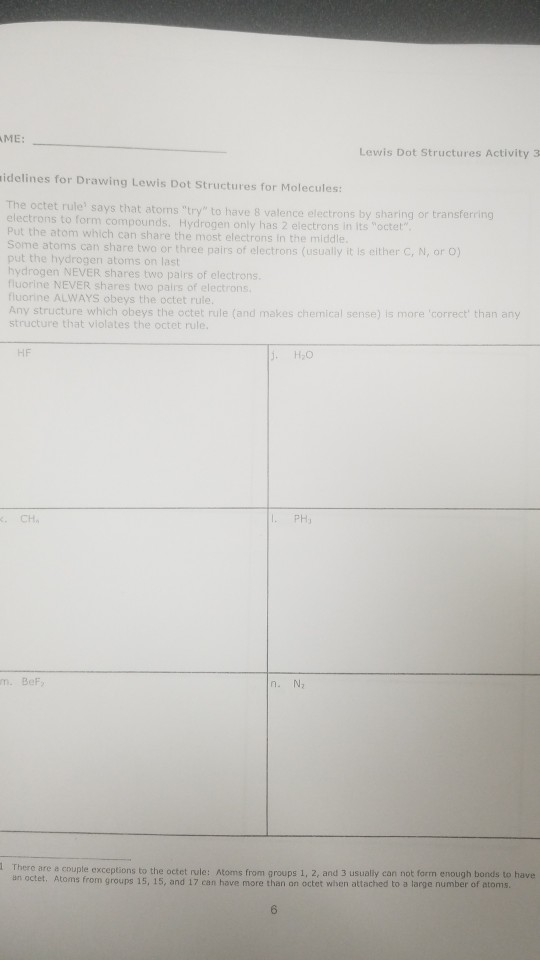
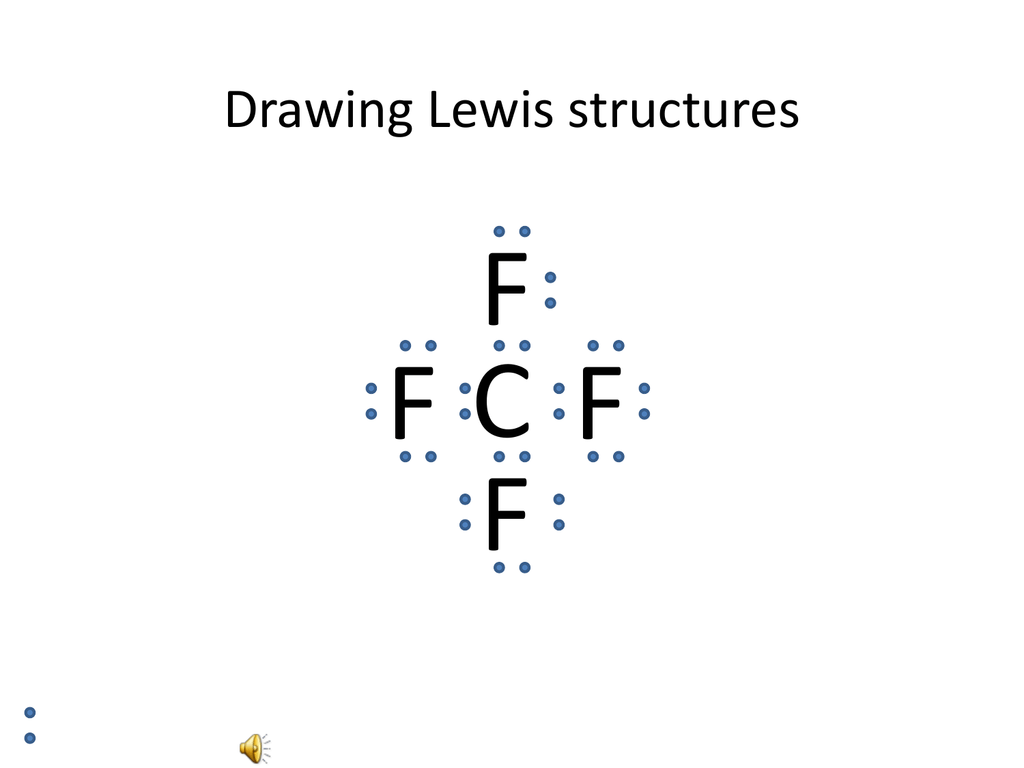
How To Draw Lewis Dot Structures Of Molecules
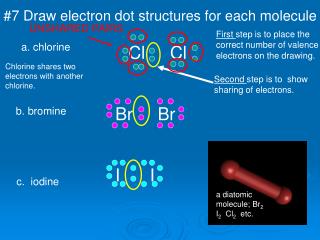
Draw the lewis dot structure for each atom of the molecule to show how many valence electrons are present in each atom of the molecule.
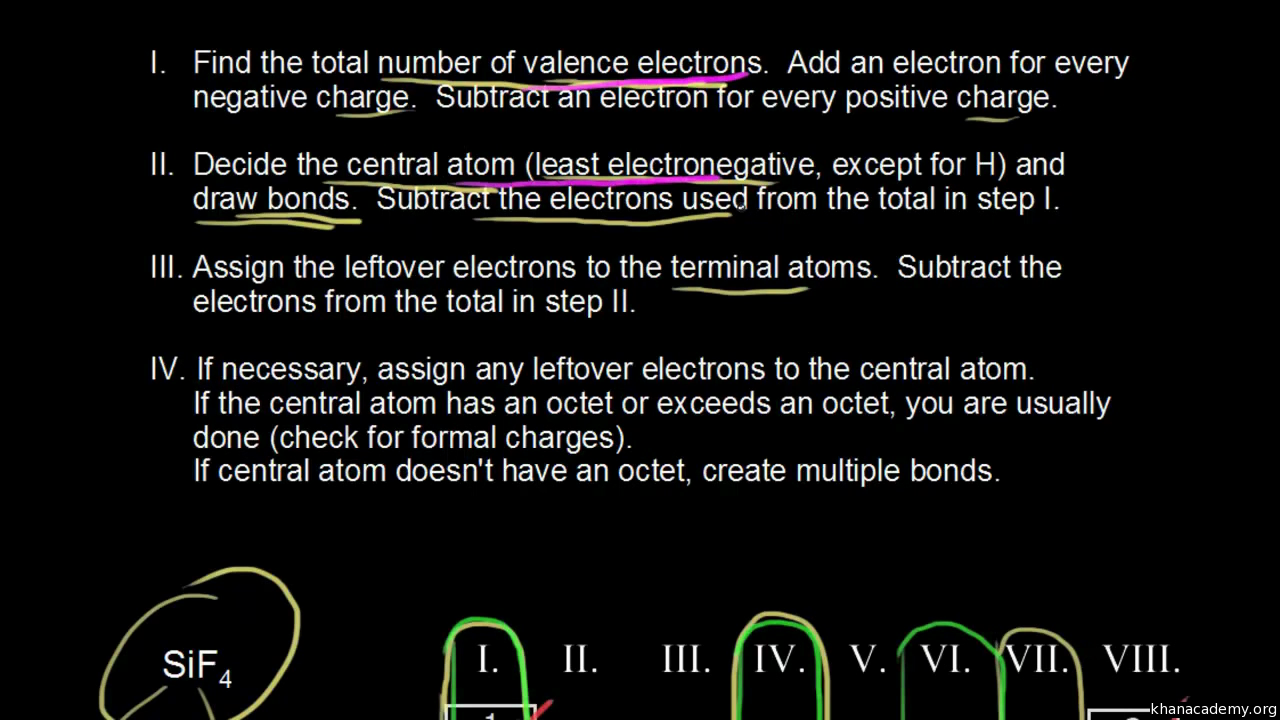
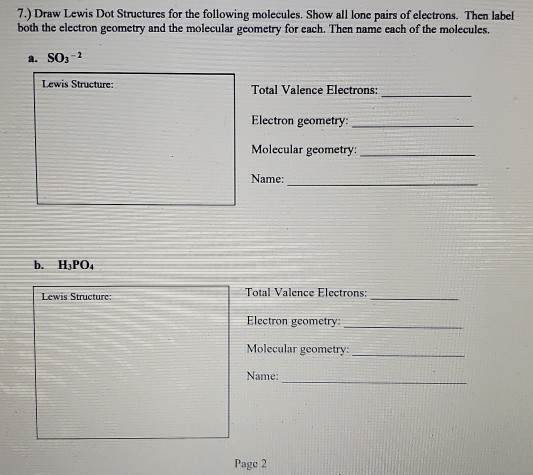
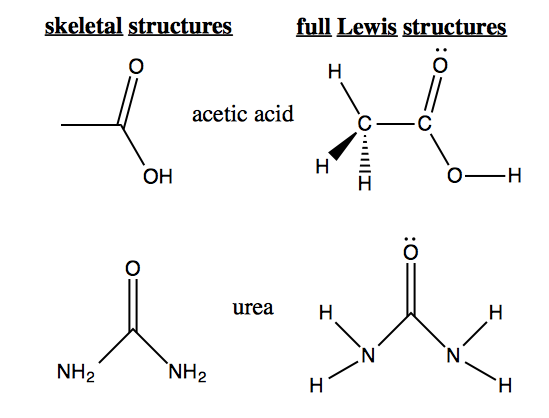
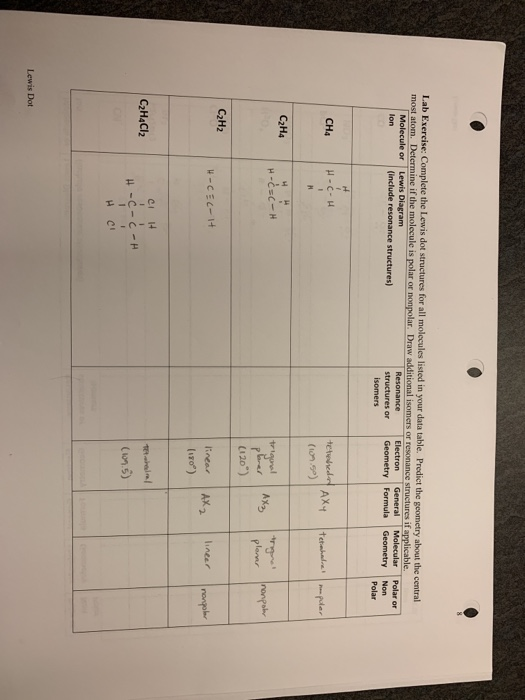
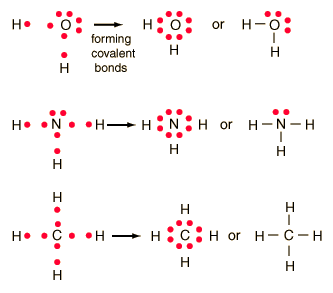
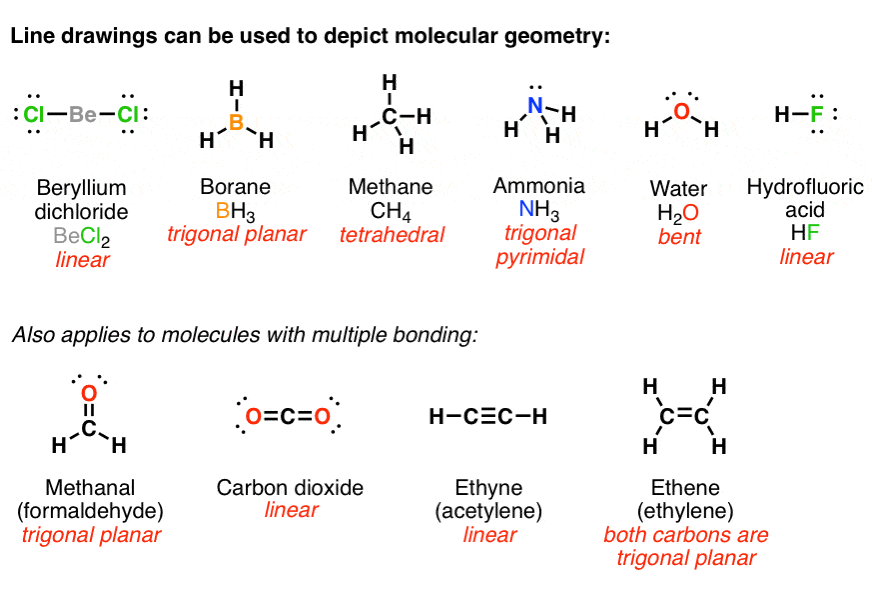
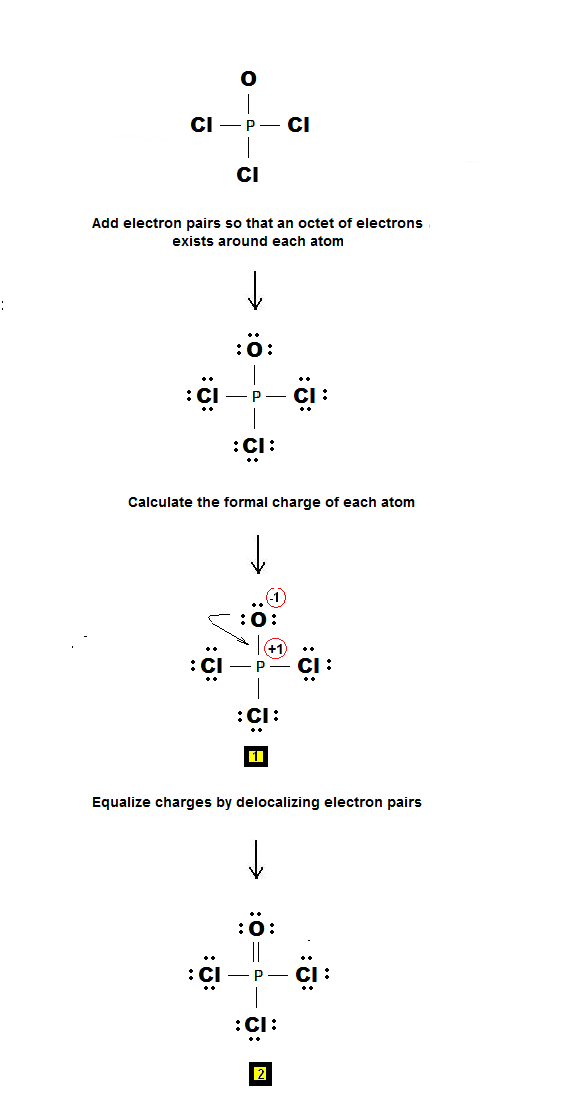
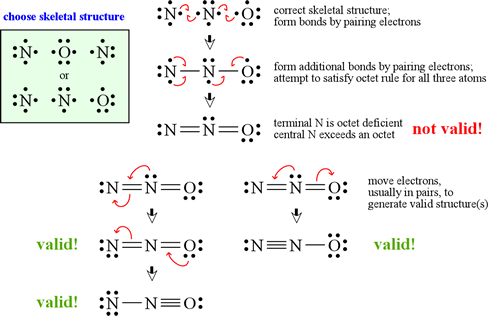
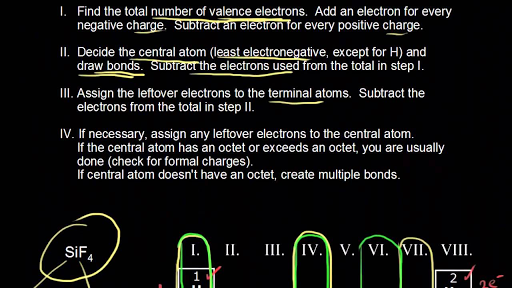
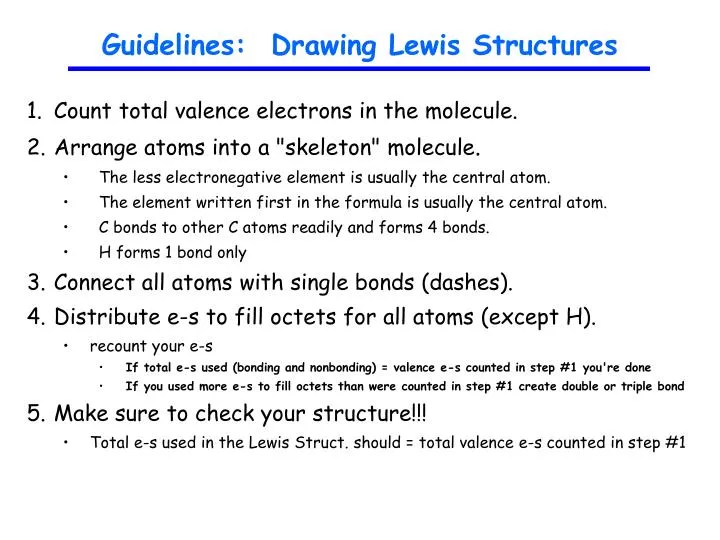
How to draw lewis dot structures of molecules. In lewis dot structure only valence electrons are used for making of the. In this packet a video and practice problems review how to draw lewis dot diagrams for complex molecules and ions including those containing double and triple bonds those. In this step add up the total number of valence electrons from all the atoms in the molecule. The following procedure can be used to construct lewis electron structures for more complex molecules and ions.
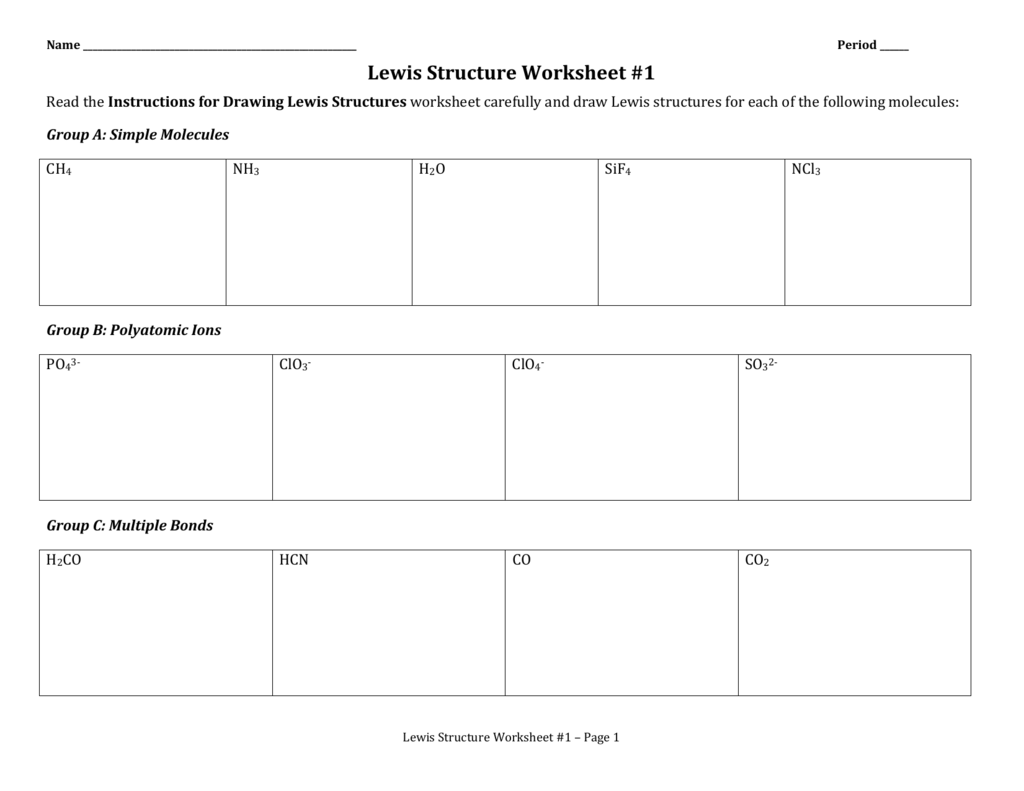
Dont forget to include any positive or. Draw the simple structure skeleton structure of the compound by connecting everything with single bonds only. Add together the valence electrons from each atom. A step by step guide step 1.
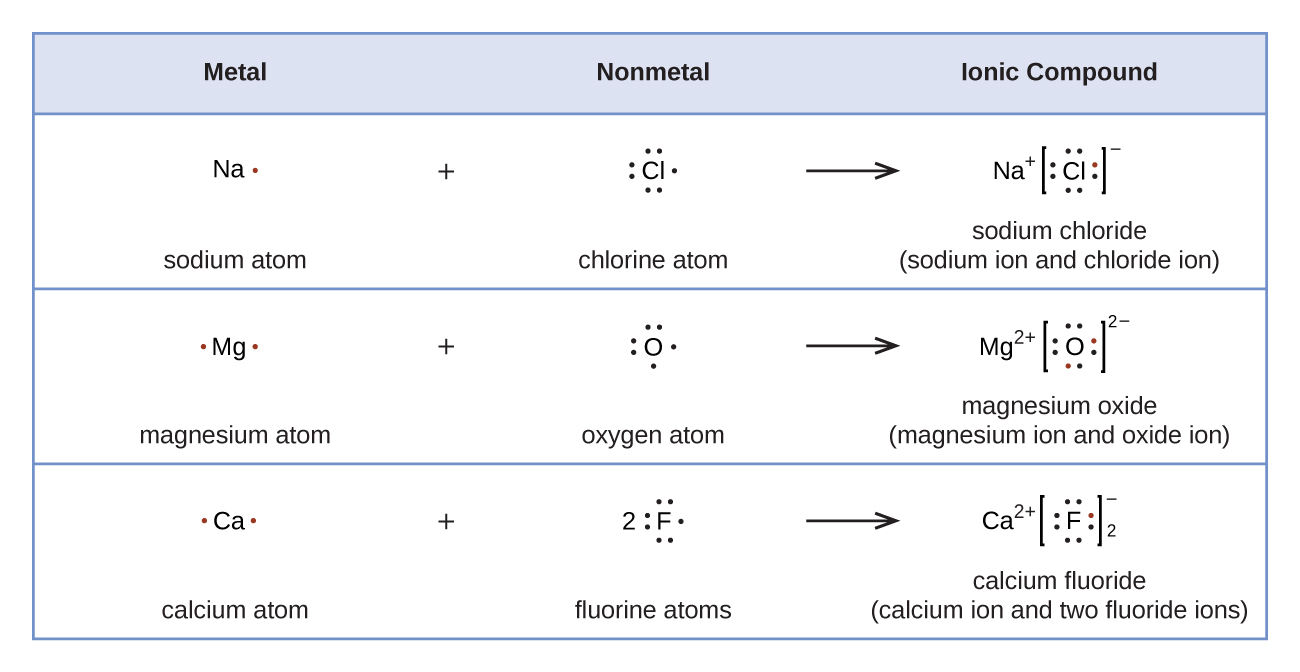
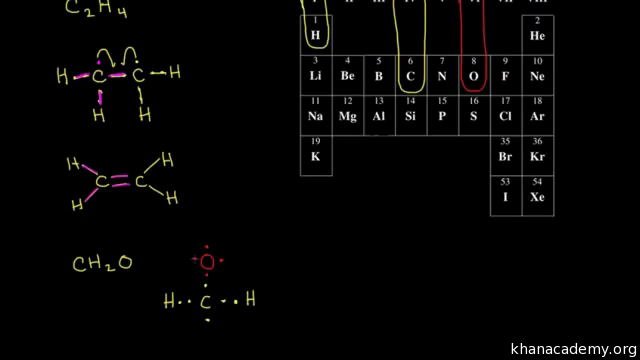
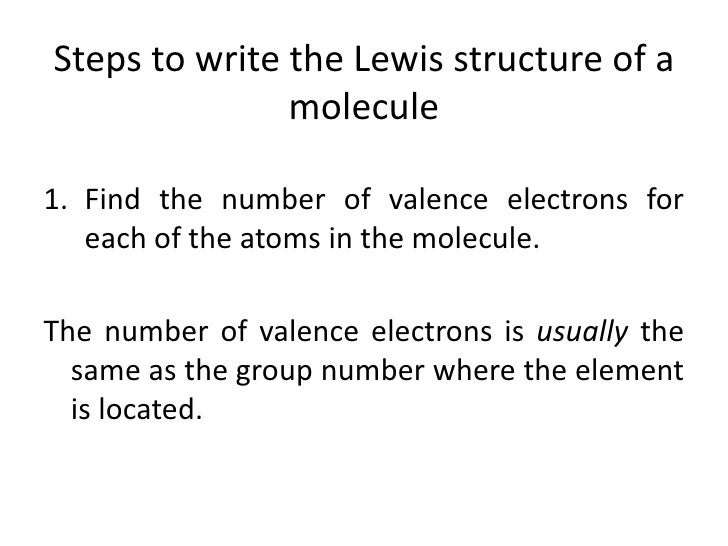
Count the total valence electrons. Add up the total number of valence electrons found in the entire compound. For example the carbon atom in co 2 in carbon dioxide has four valence electrons and the oxygen atoms have six valence electrons. These instructions outline the kelter strategy to draw lewis structuresfor molecules.
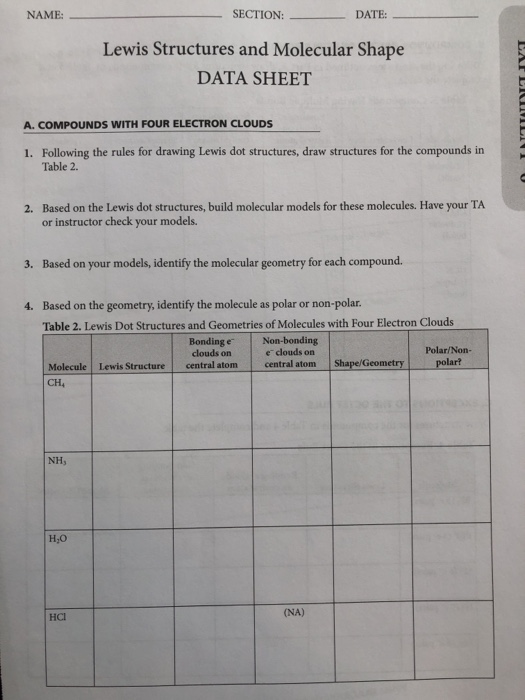
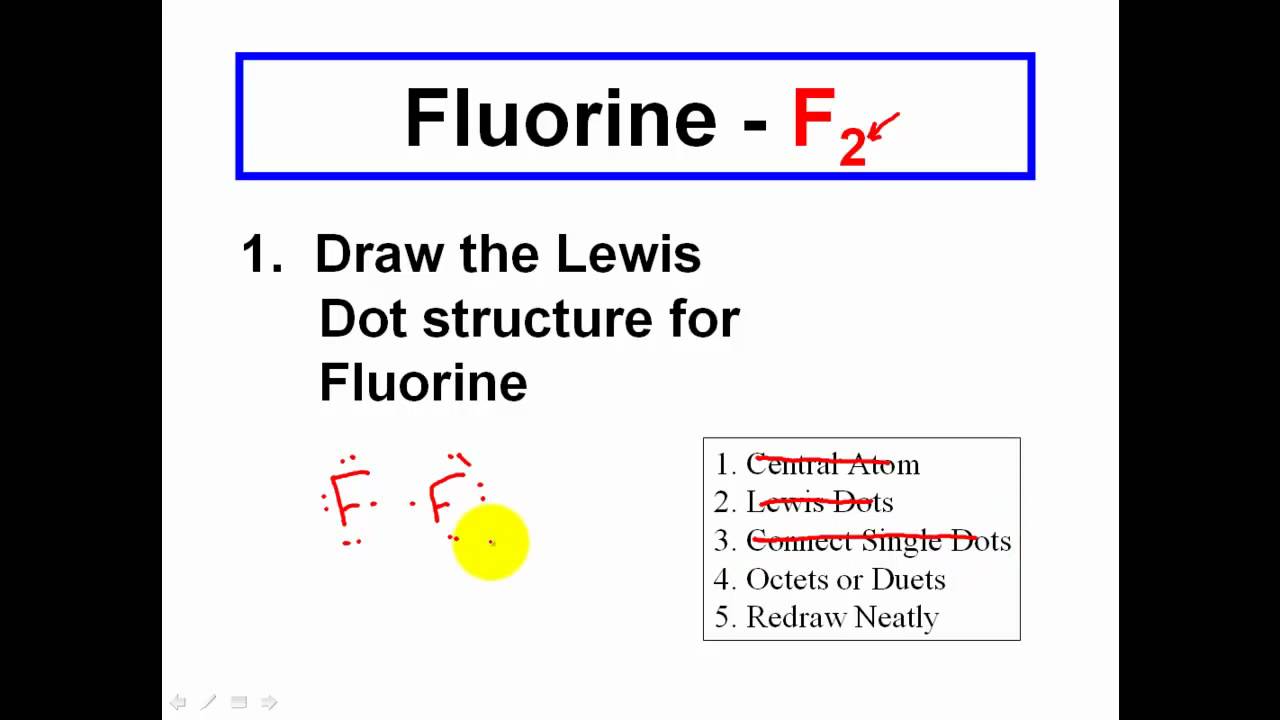
Make a skeleton of the structure. Find the total number of valence electrons. Drawing the lewis structure yourself can make understanding and interpreting lewis structures easier and therefore breaking down the creation of lewis structure into a few simple steps is advised. How to draw lewis dot structure.
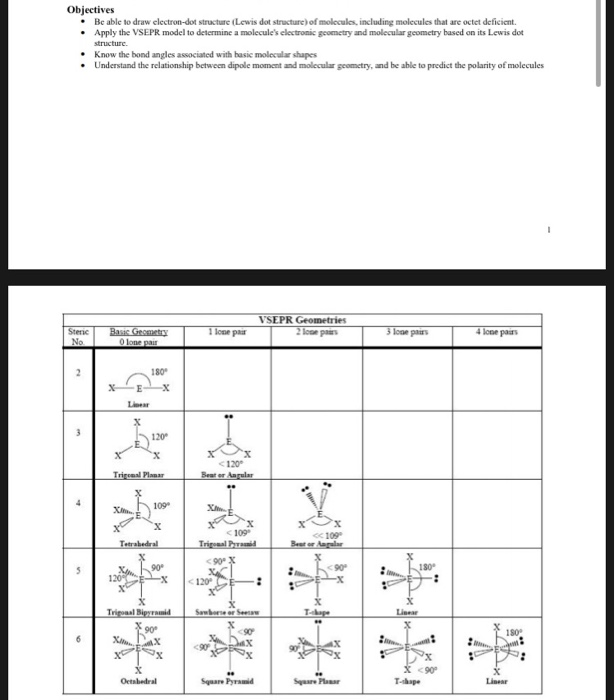
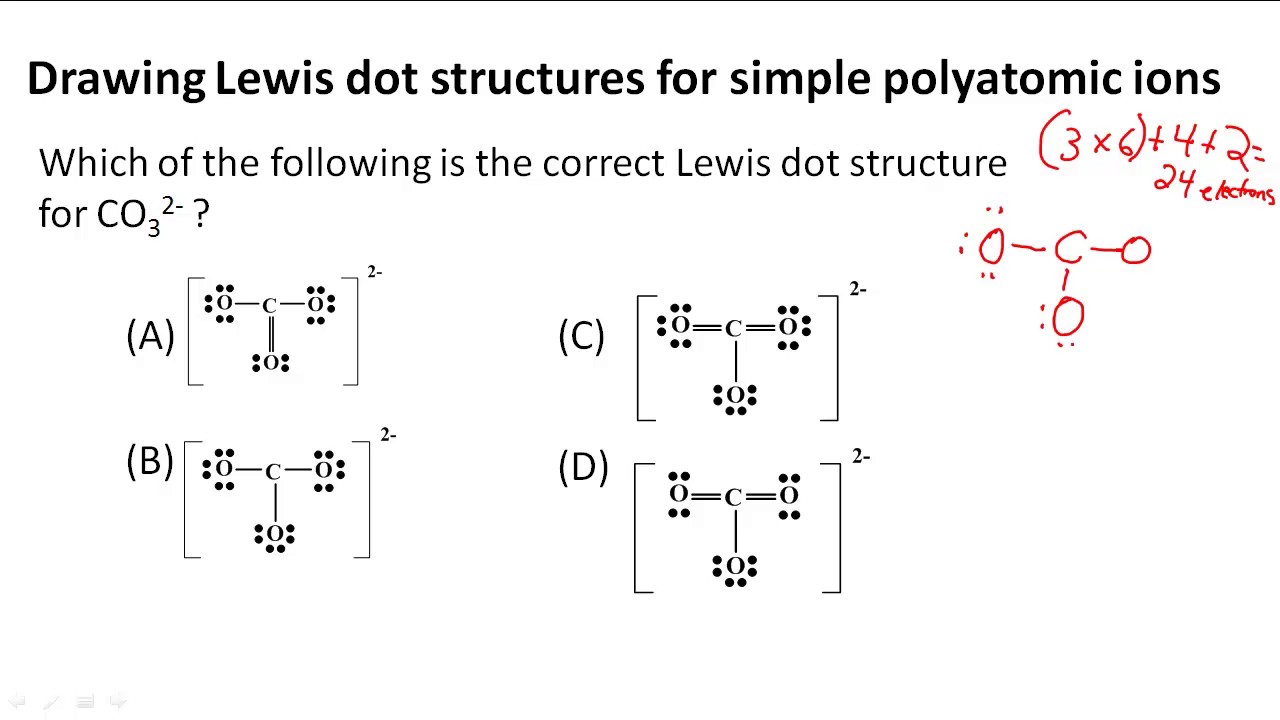
The first part of creating a lewis structure is analyzing the molecule as a whole and counting how many valence electrons the molecule possesses in. Constructing lewis electron structures. For selecting the central atom we should have a good knowledge of the. To understand how to draw a lewis dot structure for a polyatomic ion.
Draw lewis structures for covalent compounds. Determine the total number of valence electrons in the molecule or ion. First the total number of valence electrons present in the molecule is calculated by adding the individual valencies of each atom. Find the number of electrons needed to make the atoms happy.






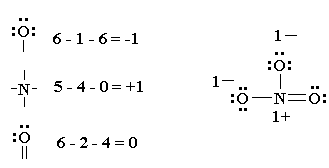




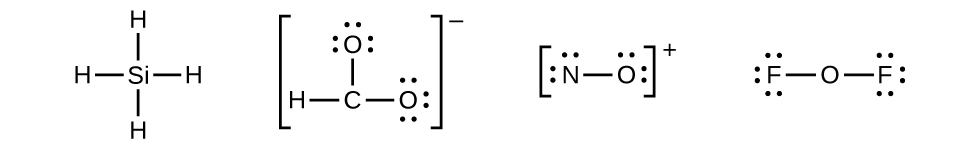

:max_bytes(150000):strip_icc()/lewisnitrite-56a128825f9b58b7d0bc90cf.jpg)




/lewis-fc84e3f1452e4aacb2fe023cfff2fa08.jpg)

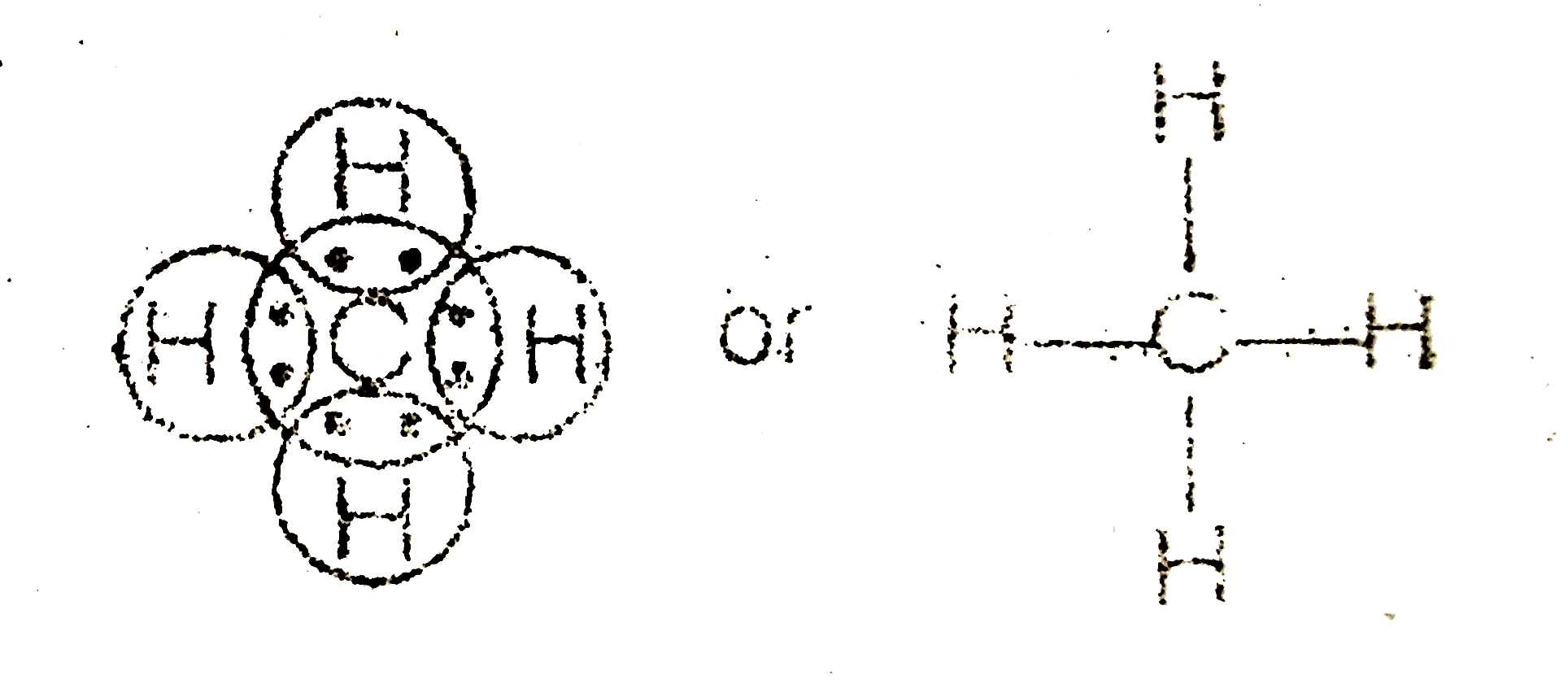
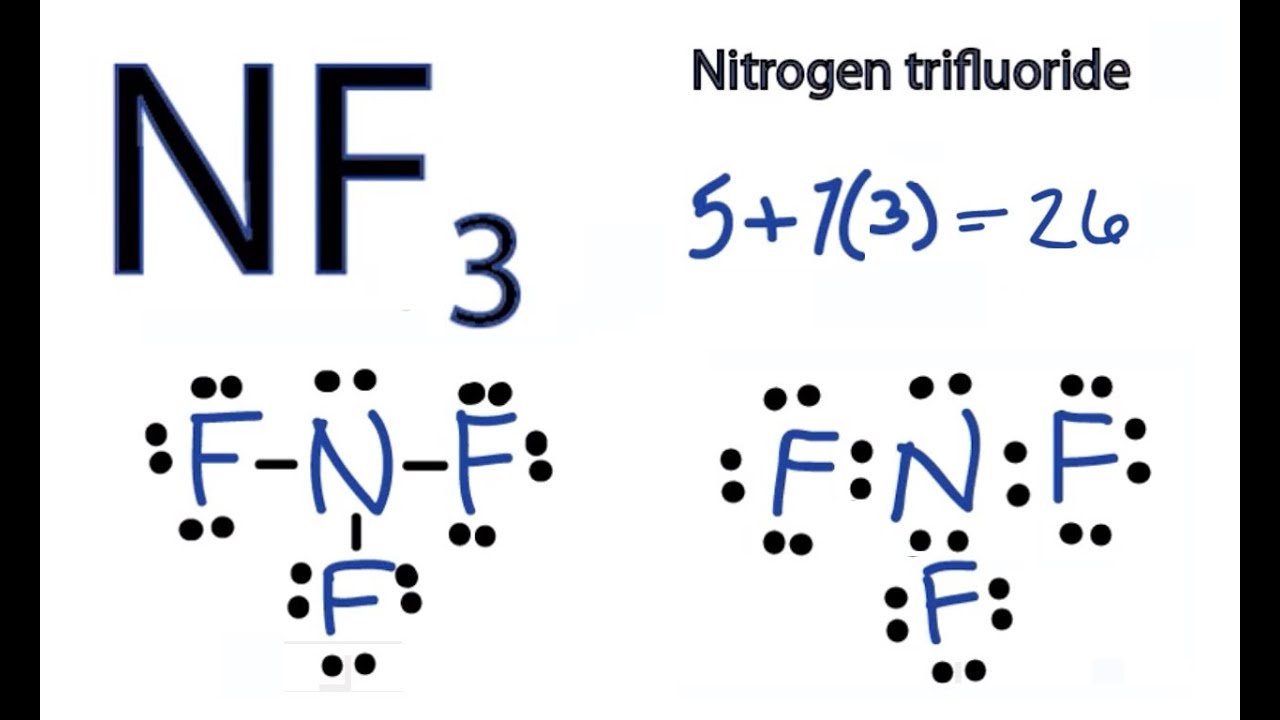


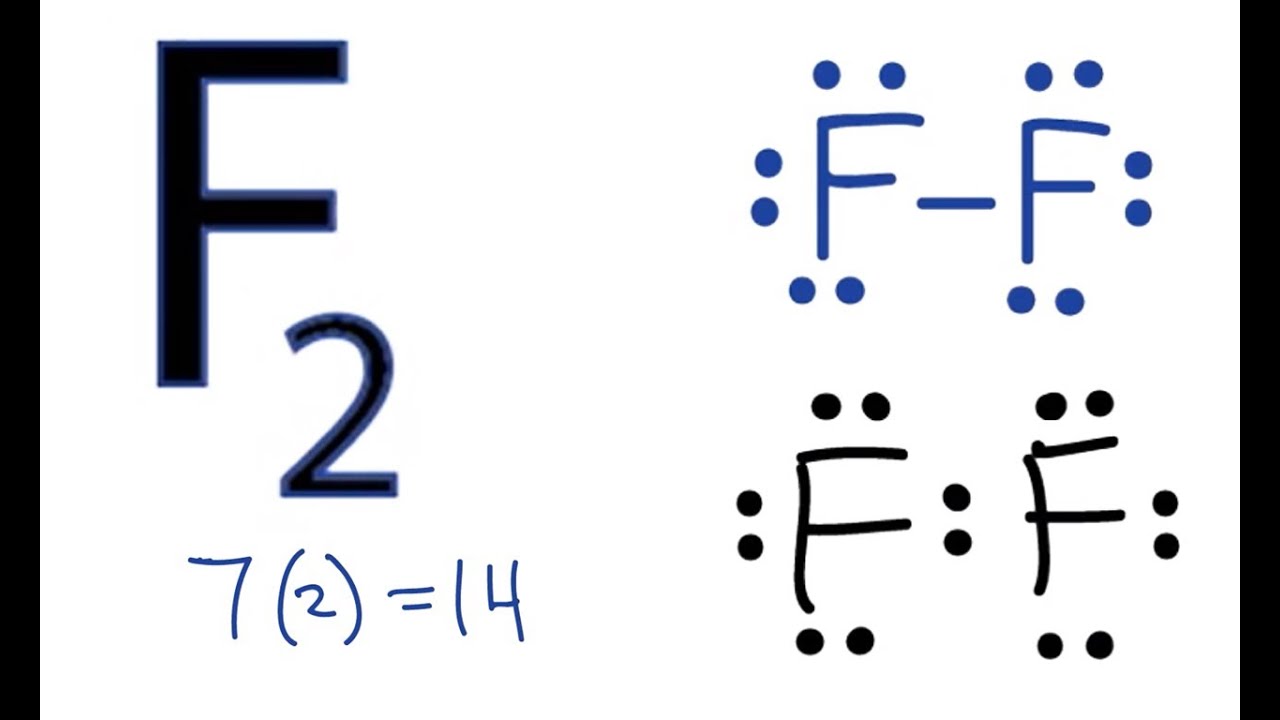
/Lewis-dot-structure-58e5390f3df78c5162b4c3db.jpg)

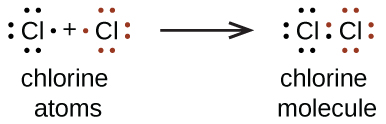
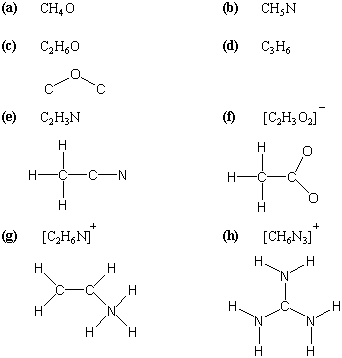












:max_bytes(150000):strip_icc()/ICl3_LD-56a12a2b3df78cf77268034c.png)







:max_bytes(150000):strip_icc()/ScreenShot2018-11-19at11.40.52PM-5bf3909a46e0fb00510dbd6d.png)